Droplet microfluidics for single-cell studies: a frontier in ecological understanding of microbiomes
- PMID: 40699006
- PMCID: PMC12342979
- DOI: 10.1093/femsre/fuaf032
Droplet microfluidics for single-cell studies: a frontier in ecological understanding of microbiomes
Abstract
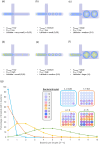
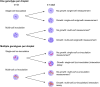
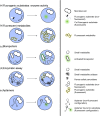
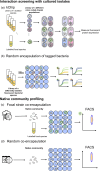
Recent advances in single-cell technologies have profoundly impacted our understanding of microbial communities-shedding light on cell-to-cell variability in gene expression, regulatory dynamics, and metabolic potential. These approaches have shown that microbial populations are more heterogeneous and functionally complex than previously thought. However, direct probing of single-cell physiology-arguably more ecologically relevant by focusing on functional traits such as growth, metabolic activity, and enzymatic activity-remains underexplored. Droplet microfluidics provides a practical and high-throughput approach to address this gap, allowing functional characterization of individual microbial cells within complex communities and offering new opportunities to study ecological processes at high resolution. In this review, we look at the state of droplet microfluidics for single-cell microbial ecology. We revisit the fundamentals of microbial droplet workflows, we overview the current capabilities of droplet microfluidics that exist for microbial ecology and we look at the phenomena these workflows have uncovered and understanding they have generated. Finally, we integrate these capabilities to envision future droplet workflows that could enhance our understanding of single-cell physiology and discuss the fundamental limitations that go together with the droplet format.
Keywords: bacterial interactions; cultivation; double emulsion; droplet microfluidics; functionality; microbial ecology; microbiology; single-cell technologies.
© The Author(s) 2025. Published by Oxford University Press on behalf of FEMS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Ultrahigh-throughput screening of environmental bacteria for proteolytic activity using droplet-based microfluidics.Appl Environ Microbiol. 2025 Jul 23;91(7):e0010925. doi: 10.1128/aem.00109-25. Epub 2025 Jun 13. Appl Environ Microbiol. 2025. PMID: 40511932 Free PMC article.
-
Survivor, family and professional experiences of psychosocial interventions for sexual abuse and violence: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2022 Oct 4;10(10):CD013648. doi: 10.1002/14651858.CD013648.pub2. Cochrane Database Syst Rev. 2022. PMID: 36194890 Free PMC article.
-
How lived experiences of illness trajectories, burdens of treatment, and social inequalities shape service user and caregiver participation in health and social care: a theory-informed qualitative evidence synthesis.Health Soc Care Deliv Res. 2025 Jun;13(24):1-120. doi: 10.3310/HGTQ8159. Health Soc Care Deliv Res. 2025. PMID: 40548558
-
The Lived Experience of Autistic Adults in Employment: A Systematic Search and Synthesis.Autism Adulthood. 2024 Dec 2;6(4):495-509. doi: 10.1089/aut.2022.0114. eCollection 2024 Dec. Autism Adulthood. 2024. PMID: 40018061 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

