Time to recovery following open and endoscopic carpal tunnel decompression: meta-analysis
- PMID: 40699057
- PMCID: PMC12284922
- DOI: 10.1093/bjsopen/zraf085
Time to recovery following open and endoscopic carpal tunnel decompression: meta-analysis
Abstract
Background: Carpal tunnel release (CTR) can be performed using either an open or endoscopic approach. The patient recovery trajectories remain poorly understood. This study aimed to define and compare patient-reported recovery following unilateral open and endoscopic CTR.
Methods: A PRISMA-compliant, preregistered (CRD42023427718) systematic review was conducted, searching PubMed, Embase, and Cochrane databases on 4 July 2023 and 21 August 2024. Studies were included if they reported recovery data (patient-reported outcome measures (PROMs)) at predefined time points for adults undergoing unilateral CTR. Boston Carpal Tunnel Questionnaire and Quick Disabilities of Arm, Shoulder, and Hand scores were extracted. Standardized mean change (SMC) scores from baseline were pooled using random-effects meta-analysis. An innovative modification of the National Institutes of Health quality assessment tools was used to evaluate the risk of bias.
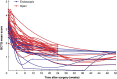
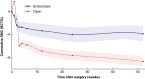
Results: In all, 49 studies were included (4546 participants included in the analysis; 3137 open CTR, 1409 endoscopic CTR). Both approaches improved PROM scores over 12 weeks, with early (4-week) outcomes strongly correlating (>0.89) with later (12-week) outcomes. Symptoms continued improving up to 104 weeks. At 1 week, open CTR showed symptomatic deterioration (SMC 10.29; 95% confidence interval (c.i.) 6.35 and 14.21 respectively), comparatively, endoscopic CTR demonstrated an improvement (SMC -2.83; 95% c.i. -7.80 and 2.14 respectively). By 2 weeks, symptom severity remained slightly worse in open CTR, but confidence intervals overlapped from week 3 and thereafter open CTR showed greater symptomatic improvement. Most studies had a high risk of bias and measured outcomes too infrequently for a granular comparison.
Conclusions: Patient-reported recovery trajectories for CTR can inform patient counselling and future research. Endoscopic CTR may result in fewer symptoms in the first 2 weeks, but open CTR may offer comparable or potentially greater improvement thereafter. Future trials with high-frequency PROM capture should prioritize early (first 3 weeks) and long-term (≥24 weeks) outcomes.
Keywords: orthopaedics; plastic surgery.
© The Author(s) 2025. Published by Oxford University Press on behalf of BJS Foundation Ltd.
Figures
References
-
- Gerritsen AA, Uitdehaag BM, Geldere D, Scholten RJ, Vet HC, Bouter LM. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg 2001;10:1285–1295 - PubMed
-
- MacDonald E, Rea PM. A systematic review of randomised control trials evaluating the efficacy and safety of open and endoscopic carpal tunnel release. Adv Exp Med Biol 2022;1356:141–172 - PubMed
-
- Thoma A, Veltri K, Haines T, Duku E. A systematic review of reviews comparing the effectiveness of endoscopic and open carpal tunnel decompression. Plast Reconstr Surg 2004;4:1184–1191 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

