Biological evaluation of a glucose-based boron carrier as a potential agent for boron neutron capture therapy
- PMID: 40699179
- PMCID: PMC12496008
- DOI: 10.1002/ijc.70054
Biological evaluation of a glucose-based boron carrier as a potential agent for boron neutron capture therapy
Abstract
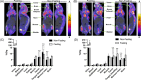
Boron neutron capture therapy (BNCT) is an innovative radiation oncology approach that targets tumors selectively, minimizing damage to healthy tissues through high-linear-energy-transfer particles released during the boron neutron capture reaction. Current boron carriers like sodium mercaptoundecahydrododecaborate (BSH) and L-p-boronophenylalanine (BPA) face limitations in specificity and solubility. Our recently developed 6-O-(o-carboranylmethyl)-d-glucopyranose (B-Glc) shows promise as an alternative, demonstrating strong interactions with glucose transporters in human head and neck squamous cell carcinoma (HNSCC) CAL 27 cells in vitro. This study aims to extend in vitro investigations to three additional patient-derived human HNSCC cell lines (UT-SCC-14, UT-SCC-28, and UT-SCC-42B) and to further evaluate in vivo pharmacokinetics in selected HNSCC tumor xenografts. The B-Glc showed superior uptake and favorable kinetic parameters compared to BPA and BSH in all tested cell lines. Initial positron emission tomography imaging using [18F]fluoro-2-deoxy-d-glucose ([18F]FDG) radiotracer confirmed increased glucose uptake in CAL 27 and UT-SCC-14 tumors in vivo, supported by glucose transporter 1 (GLUT1) expression observed in tumor section immunohistochemistry. Biodistribution studies of the B-Glc (75 mg/kg dose) revealed no significant impact of blood glucose levels on tumor uptake, with peak boron accumulation at 15-30 min post-injection, comparable uptake to the clinical BPA-fructose complex (400 mg/kg dose) performance at 60 min, achieving the required tumor boron concentration (>20 ppm) for effective BNCT. Overall, this study underscores an advancement in targeted BNCT, highlighting B-Glc as an effective GLUT1-targeting carrier for enhanced therapeutic outcome in HNSCC and the potential to use [18F]FDG as a companion diagnostic for the glucoconjugate.
Keywords: BNCT; GLUT1; boron delivery agents; boron neutron capture therapy; drug delivery; glucoconjugate; glucose transporter 1.
© 2025 The Author(s). International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


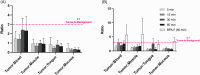
References
-
- Hatanaka H, Nakagawa Y. Clinical results of long‐surviving brain tumor patients who underwent boron neutron capture therapy. International Journal of Radiation Oncology*Biology*Physics. 1994;28:1061‐1066. - PubMed
-
- Pozzi ECC, Cardoso JE, Colombo LL, et al. Boron neutron capture therapy (BNCT) for liver metastasis: therapeutic efficacy in an experimental model. Radiat Environ Biophys. 2012;51:331‐339. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

