Cytology-Radiology Correlation Series: Thyroid cytopathology
- PMID: 40699188
- PMCID: PMC12285623
- DOI: 10.1002/cncy.70028
Cytology-Radiology Correlation Series: Thyroid cytopathology
Abstract
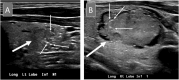
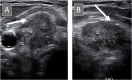
Thyroid ultrasound is typically the first step in the workup of thyroid nodules. Ultrasonographic features of thyroid nodules can be used to evaluate their risk of malignancy using risk stratification systems to determine whether a nodule is suspicious enough to warrant a more invasive fine-needle aspiration (FNA) for further evaluation. For this review, the authors described and compared two major risk stratification systems, the American Thyroid Association classification system and the American College of Radiology Thyroid Imaging Reporting and Data System, and explored corresponding ultrasound and cytology findings in the thyroid for commonly encountered entities in cytopathology practice.
Keywords: cytology; cytopathology; radiology; risk; thyroid; ultrasound.
© 2025 The Author(s). Cancer Cytopathology published by Wiley Periodicals LLC on behalf of American Cancer Society.
Conflict of interest statement
Benjamin Wildman‐Tobriner reports personal/consulting fees from Merck outside the submitted work. The remaining authors disclosed no conflicts of interest.
Figures





















Similar articles
-
Does a higher American College of Radiology Thyroid Imaging Reporting and Data System (ACR TI-RADS) score forecast an increased risk of malignancy? A correlation study of ACR TI-RADS with FNA cytology in the evaluation of thyroid nodules.Cancer Cytopathol. 2020 Jul;128(7):470-481. doi: 10.1002/cncy.22254. Epub 2020 Feb 20. Cancer Cytopathol. 2020. PMID: 32078249
-
The additive value of real-time elastography to thyroid ultrasound in detecting papillary carcinoma in nodules over 20 mm in diameter.Endocrine. 2025 Jul;89(1):177-185. doi: 10.1007/s12020-025-04248-1. Epub 2025 Apr 24. Endocrine. 2025. PMID: 40274686
-
Diagnostic Performance of Six Ultrasound Risk Stratification Systems for Thyroid Nodules: A Systematic Review and Network Meta-Analysis.AJR Am J Roentgenol. 2023 Jun;220(6):791-803. doi: 10.2214/AJR.22.28556. Epub 2023 Feb 8. AJR Am J Roentgenol. 2023. PMID: 36752367
-
Evaluation of concordance between the Bethesda System for Reporting Thyroid Cytopathology 2023 (TBSRTC) and ACR-TIRADS at a tertiary care center in Gujarat.Indian J Pathol Microbiol. 2025 Apr 1;68(2):338-343. doi: 10.4103/ijpm.ijpm_165_24. Epub 2025 Mar 13. Indian J Pathol Microbiol. 2025. PMID: 40079897
-
Evidence-based assessment of the role of ultrasonography in the management of benign thyroid nodules.World J Surg. 2008 Jul;32(7):1253-63. doi: 10.1007/s00268-008-9494-z. World J Surg. 2008. PMID: 18311500
References
-
- Kant R, Davis A, Verma V. Thyroid nodules: advances in evaluation and management. Am Fam Physician. 2020;102(5):298‐304. - PubMed
-
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1‐133. doi: 10.1089/thy.2015.0020 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

