Bioengineering approaches to trained immunity: Physiologic targets and therapeutic strategies
- PMID: 40699210
- PMCID: PMC12286607
- DOI: 10.7554/eLife.106339
Bioengineering approaches to trained immunity: Physiologic targets and therapeutic strategies
Abstract
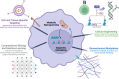
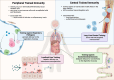
Trained immunity presents a unique target for modulating the immune response against infectious and non-infectious threats to human health. To address the unmet need for training-targeted therapies, we explore bioengineering methods to answer research questions and address clinical applications. Current challenges in trained immunity include self-propagating autoinflammatory disease, a lack of controllable cell and tissue specificity, and the unintentional induction of training by known drugs and diseases. The bioengineering tools discussed in this review (nanotherapeutics, biomechanical modulation, cellular engineering, and machine learning) could address these challenges by providing additional avenues to modulate and interrogate trained immunity. The preferential activation of peripheral or central training has not yet been achieved and could be accessed using nanoparticle systems. Targeted delivery of training stimuli using nanocarriers can enrich the response in various cell and organ systems, while also selectively activating peripheral training in the local tissues or central trained immunity in bone marrow progenitor cells. Beyond chemical- or pathogen-based activation of training, force-based cues, such as interaction with mechanoreceptors, can induce trained phenotypes in many cell types. Mechanotransduction influences immune cell activation, motility, and morphology and could be harnessed as a tool to modulate training states in next-generation therapies. For known genetic and epigenetic mediators of trained immunity, cellular engineering could precisely activate or deactivate programs of training. Genetic engineering could be particularly useful in generating trained cell-based therapies like chimeric antigen receptor (CAR) macrophages. Finally, machine learning models, which are rapidly transforming biomedical research, can be employed to identify signatures of trained immunity in pre-existing datasets. They can also predict protein targets for previously identified inducers of trained immunity by modeling drug-protein or protein-protein interactions in silico. By harnessing the modular techniques of bioengineering for applications in trained immunity, training-based therapies can be more efficiently translated into clinical practice.
Keywords: bioengineering; biomechanics; cellular engineering; immunology; inflammation; machine learning; nanotherapeutics; trained immunity.
© 2025, Knight et al.
Conflict of interest statement
HK H.R.K. and A.E.-K. are inventors on a patent disclosing small molecule inducers of trained immunity for the University of Chicago. All other authors declare no competing interests, MK, NK, HT, HM, EA, AE No competing interests declared
Figures



References
-
- Aaby P, Roth A, Ravn H, Napirna BM, Rodrigues A, Lisse IM, Stensballe L, Diness BR, Lausch KR, Lund N, Biering-Sørensen S, Whittle H, Benn CS. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? The Journal of Infectious Diseases. 2011;204:245–252. doi: 10.1093/infdis/jir240. - DOI - PubMed
-
- Abramson J, Adler J, Dunger J, Evans R, Green T, Pritzel A, Ronneberger O, Willmore L, Ballard AJ, Bambrick J, Bodenstein SW, Evans DA, Hung C-C, O’Neill M, Reiman D, Tunyasuvunakool K, Wu Z, Žemgulytė A, Arvaniti E, Beattie C, Bertolli O, Bridgland A, Cherepanov A, Congreve M, Cowen-Rivers AI, Cowie A, Figurnov M, Fuchs FB, Gladman H, Jain R, Khan YA, Low CMR, Perlin K, Potapenko A, Savy P, Singh S, Stecula A, Thillaisundaram A, Tong C, Yakneen S, Zhong ED, Zielinski M, Žídek A, Bapst V, Kohli P, Jaderberg M, Hassabis D, Jumper JM. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature. 2024;630:493–500. doi: 10.1038/s41586-024-07487-w. - DOI - PMC - PubMed
-
- Ajit J, Knight HR, Chen Q, Solanki A, Shen J, Kahn APE. Novel Non-Immunogenic Trained Immunity Inducing Small Molecule with Improved Anti-Tumor Propertie. bioRxiv. 2024 doi: 10.1101/2024.03.22.585780. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

