Single cell ICP-MS for the assessment of potential nephroprotectors against cisplatin
- PMID: 40699350
- PMCID: PMC12287176
- DOI: 10.1007/s00604-025-07383-8
Single cell ICP-MS for the assessment of potential nephroprotectors against cisplatin
Abstract
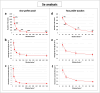
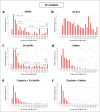
The use of cisplatin chemotherapy is often limited by the occurrence of various side effects, with renal toxicity being one of the most serious. In the present work, a single cell ICP-MS (scICP-MS) methodology was optimised to evaluate the cellular uptake of cisplatin in the presence of three potential nephroprotectors such as chitosan-stabilised selenium nanoparticles (Ch-SeNPs), selenomethionine (SeMet) and methionine (Met). Human telomerase reverse transcriptase-immortalised renal proximal tubular epithelial cells (RPTEC/TERT1) and human cervical cancer cells (HeLa) were employed with this aim. In both cell lines, a decrease in the intracellular Pt levels when using SeMet and Met as coadjuvants was revealed, involving less toxicity in renal cells but no reduction in the anticancer effect after measurement of cell viability by MTT assays. In contrast, Ch-SeNPs had no effect on the internalisation of the Pt-drug but enhanced its antitumour efficacy with no additional damage to kidney cells. This would allow decreasing cisplatin doses which would in turn reduce nephrotoxicity risk. Se determination by scICP-MS was also done to study the cell uptake of the selenocompounds, in addition to transmission electron microscopy (TEM) analysis of Ch-SeNPs internalisation. The effects of both SeMet and Ch-SeNPs were confirmed despite the Pt-drug was shown to induce a decrease in cell uptake. Results were compared by two different scICP-MS settings (a conventional introduction system and a special configuration for intact cells), as well as with the classical digestion-based bulk analysis. Our results demonstrate the potential of scICP-MS for metallomic cellular studies to improve cisplatin-based therapies.
Keywords: Cisplatin; Kidney protection; Methionine; Selenium nanoparticles; Selenomethionine; Single cell ICP-MS.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures





Similar articles
-
Metallomic evaluation of selenium nanoparticles and selenomethionine for the attenuation of cisplatin-induced nephrotoxicity.Eur J Pharm Biopharm. 2025 Jul;212:114737. doi: 10.1016/j.ejpb.2025.114737. Epub 2025 May 8. Eur J Pharm Biopharm. 2025. PMID: 40345401
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Biological activities of optimized biosynthesized selenium nanoparticles using Proteus mirabilis PQ350419 alone or combined with chitosan and ampicillin against common multidrug-resistant bacteria.Microb Cell Fact. 2025 Jul 5;24(1):159. doi: 10.1186/s12934-025-02783-0. Microb Cell Fact. 2025. PMID: 40618114 Free PMC article.
References
-
- Dos Santos NAG, Rodrigues MAC, Martins NM, Dos Santos AC (2012) Cisplatin-induced nephrotoxicity and targets of nephroprotection: an update. Arch Toxicol 86(8):1233–1250. 10.1007/s00204-012-0821-7 - PubMed
MeSH terms
Substances
Grants and funding
- FPU 14/01412/Ministerio de Ciencia, Innovación y Universidades
- FPU 13/01693/Ministerio de Ciencia, Innovación y Universidades
- PID2020-116067RB-100/AEI/10.13039/50110001103/Ministerio de Ciencia, Innovación y Universidades
- PID2020-116067RB-100/AEI/10.13039/50110001103/Ministerio de Ciencia, Innovación y Universidades
- ED431C2018/19/Xunta de Galicia
LinkOut - more resources
Full Text Sources
Miscellaneous
