Optimizing Timing for Respiratory Syncytial Virus Prevention Interventions for Infants
- PMID: 40699573
- PMCID: PMC12287852
- DOI: 10.1001/jamanetworkopen.2025.22779
Optimizing Timing for Respiratory Syncytial Virus Prevention Interventions for Infants
Abstract
Importance: The Advisory Committee on Immunization Practices and the Centers for Disease Control and Prevention recommend seasonal administration of maternal vaccine (MV) or nirsevimab to protect infants in the first year of life from respiratory syncytial virus (RSV) infections. Differences in uptake, costs, and efficacy between these agents may affect cost-effectiveness depending on the timing of administration.
Objective: To evaluate the clinical outcomes and cost-effectiveness of no intervention, MV, and nirsevimab administration during the entire RSV season and separately for each monthly birth cohort from October through February.
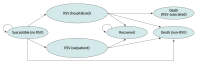
Design, setting, and participants: This economic evaluation used a Markov model to analyze cost-effectiveness from a societal perspective, applying a willingness-to-pay threshold of $150 000 per quality-adjusted life-year (QALY). Participants included infants born in the US during the RSV season. Data were accrued from October 2023 to June 2024 and analyzed from July 2024 to May 2025.
Interventions: MV, nirsevimab administration, or no intervention.
Main outcomes and measures: The primary outcome was the incremental cost-effectiveness ratio (cost per QALY gained). Clinical outcomes included the number of hospitalizations, outpatient infections, and deaths averted. Probabilistic sensitivity analyses were conducted.
Results: An estimated monthly birth cohort of 299 277 infants was included in the analysis. Compared with no intervention, MV was cost-saving for infants in the October, November, and December cohorts and cost-effective in the January but not the February cohorts ($504 517/QALY). For infants born in the combined cohort (October-February), MV was cost-effective at $19 562/QALY. Compared with MV, nirsevimab was cost-effective only in October ($67 178/QALY) and November ($88 531/QALY). During the RSV season, MV was projected to avert 45 558 outpatient visits, 7154 hospitalizations, and 12 deaths; nirsevimab was projected to avert 92 265 outpatient visits, 11 893 hospitalizations, and 19 deaths. The probability of no intervention being cost-effective during the RSV season was 19.8%; MV, 62.2%; and nirsevimab, 18.0%.
Conclusions and relevance: In this economic evaluation of RSV prevention interventions, administration of MV in the first 4 months of and throughout the viral season could be cost-effective. Across the entire RSV season, nirsevimab was cost-effective compared with MV only in October and November. Intervention use may be optimized by restricting administration to select months. Further study is needed to assess transmission dynamics to refine cost-effectiveness outcomes.
Conflict of interest statement
Figures
References
-
- Centers for Disease Control and Prevention (CDC) . Respiratory syncytial virus (RSV) surveillance. 2021. Accessed March 1, 2024. https://www.cdc.gov/surveillance/nrevss/rsv/index.html
-
- American Academy of Pediatrics Committee on Infectious Diseases; American Academy of Pediatrics Bronchiolitis Guidelines Committee . Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134(2):e620-e638. doi: 10.1542/peds.2014-1666 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical