Expression and Biological Activity Analysis of Recombinant Fibronectin3 Protein in Bacillus subtilis
- PMID: 40700133
- PMCID: PMC12286206
- DOI: 10.3390/biotech14030051
Expression and Biological Activity Analysis of Recombinant Fibronectin3 Protein in Bacillus subtilis
Abstract
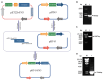
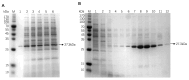
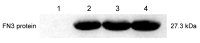
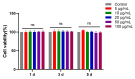
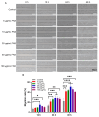
Fibronectin (FN), a primary component of the extracellular matrix (ECM), features multiple structural domains closely linked to various cellular behaviors, including migration, spreading, adhesion, and proliferation. The FN3 domain, which contains the RGD sequence, is critical in tissue repair because it enables interaction with integrin receptors on the cell surface. However, the large molecular weight of wild-type FN presents challenges for its large-scale production through heterologous expression. Therefore, this study focused on cloning the FN3 functional domain of full-length FN for expression and validation. This study selected Bacillus subtilis as the expression host due to its prominent advantages, including efficient protein secretion, absence of endotoxins, and minimal codon bias. The recombinant vector pHT43-FN3 was successfully constructed through homologous recombination technology and transformed into Bacillus subtilis WB800N. The FN3 protein was successfully expressed after induction with IPTG. Following purification of the recombinant FN protein using a His-tag nickel column, SDS-PAGE analysis showed that the molecular weight of FN3 was approximately 27.3 kDa. Western blot analysis confirmed the correct expression of FN3, and the BCA protein assay kit determined a protein yield of 5.4 mg/L. CCK8 testing demonstrated the good biocompatibility of FN3. In vitro cell experiments showed that FN3 significantly promoted cell migration at a 20 μg/mL concentration and enhanced cell adhesion at 10 μg/mL. In summary, this study successfully utilized Bacillus subtilis to express the FN3 functional domain peptide from FN protein and has validated its ability to promote cell migration and adhesion. These findings not only provide a strategy for the expression of FN protein in B. subtilis, but also establish an experimental foundation for the potential application of FN3 protein in tissue repair fields such as cutaneous wound healing and cartilage regeneration.
Keywords: Bacillus subtilis; cell adhesion; cell migration; recombinant fibronectin3 protein.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Systemic Inflammatory Response Syndrome.2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31613449 Free Books & Documents.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
