Proteomic Profiling Reveals Novel Molecular Insights into Dysregulated Proteins in Established Cases of Rheumatoid Arthritis
- PMID: 40700276
- PMCID: PMC12286002
- DOI: 10.3390/proteomes13030032
Proteomic Profiling Reveals Novel Molecular Insights into Dysregulated Proteins in Established Cases of Rheumatoid Arthritis
Abstract
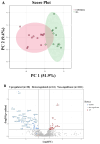
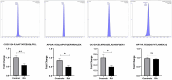
Background: Rheumatoid arthritis (RA) is a chronic autoimmune disorder that predominantly affects synovial joints, leading to inflammation, pain, and progressive joint damage. Despite therapeutic advancements, the molecular basis of established RA remains poorly defined. Methods: In this study, we conducted an untargeted plasma proteomic analysis using two-dimensional differential gel electrophoresis (2D-DIGE) and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) in samples from RA patients and healthy controls in the discovery phase. Results: Significantly (ANOVA, p ≤ 0.05, fold change > 1.5) differentially abundant proteins (DAPs) were identified. Notably, upregulated proteins included mitochondrial dicarboxylate carrier, hemopexin, and 28S ribosomal protein S18c, while CCDC124, osteocalcin, apolipoproteins A-I and A-IV, and haptoglobin were downregulated. Receiver operating characteristic (ROC) analysis identified CCDC124, osteocalcin, and metallothionein-2 with high diagnostic potential (AUC = 0.98). Proteins with the highest selected frequency were quantitatively verified by multiple reaction monitoring (MRM) analysis in the validation cohort. Bioinformatic analysis using Ingenuity Pathway Analysis (IPA) revealed the underlying molecular pathways and key interaction networks involved STAT1, TNF, and CD40. These central nodes were associated with immune regulation, cell-to-cell signaling, and hematological system development. Conclusions: Our combined proteomic and bioinformatic approaches underscore the involvement of dysregulated immune pathways in RA pathogenesis and highlight potential diagnostic biomarkers. The utility of these markers needs to be evaluated in further studies and in a larger cohort of patients.
Keywords: 2D DIGE; MALDI-TOF; biomarker; metallothionein-2; osteocalcin; plasma proteomics; rheumatoid arthritis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

