Teprotumumab for Thyroid Eye Disease: Mechanism, Clinical Efficacy, and Current Challenges
- PMID: 40700295
- PMCID: PMC12286191
- DOI: 10.3390/antib14030055
Teprotumumab for Thyroid Eye Disease: Mechanism, Clinical Efficacy, and Current Challenges
Abstract
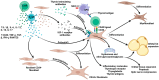
Thyroid eye disease (TED) is a complex autoimmune disorder characterized by orbital inflammation and tissue remodeling. Teprotumumab, a fully human monoclonal antibody targeting insulin-like growth factor-1 receptor (IGF-1R), represents a significant breakthrough in TED treatment. This review comprehensively analyzes the therapeutic role of teprotumumab in TED management. Mechanistically, teprotumumab inhibits the IGF-1R/TSHR signaling complex, thereby reducing orbital fibroblast differentiation and inflammatory responses. Phase II and III clinical trials have demonstrated its remarkable efficacy in reducing proptosis and improving clinical activity scores, with the benefits extending to both active and chronic TED cases. Real-world studies have validated these findings further and expanded its potential applications to various clinical scenarios, including dysthyroid optic neuropathy and steroid-resistant cases. However, several challenges remain. These include treatment-related adverse effects such as hyperglycemia and hearing impairment, with emerging evidence suggesting ethnic variations in susceptibility. The high cost of treatment poses significant accessibility barriers, while limited long-term follow-up data and potential disease recurrence necessitate further investigation. This review synthesizes the current evidence to inform clinical decision-making and highlights areas requiring additional research to optimize teprotumumab's therapeutic application in TED management.
Keywords: Graves’ disease; clinical efficacy; insulin-like growth factor-1 receptor; orbital fibroblasts; teprotumumab; thyroid eye disease.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Thyroid eye disease (Graves' orbitopathy): clinical presentation, epidemiology, pathogenesis, and management.Lancet Diabetes Endocrinol. 2025 Jul;13(7):600-614. doi: 10.1016/S2213-8587(25)00066-X. Epub 2025 May 2. Lancet Diabetes Endocrinol. 2025. PMID: 40324443 Review.
-
Long-Term Efficacy of Teprotumumab in Thyroid Eye Disease: Follow-Up Outcomes in Three Clinical Trials.Thyroid. 2024 Jul;34(7):880-889. doi: 10.1089/thy.2023.0656. Epub 2024 Jun 2. Thyroid. 2024. PMID: 38824618 Clinical Trial.
-
Redefining Treatment Paradigms in Thyroid Eye Disease: Current and Future Therapeutic Strategies.J Clin Med. 2025 Aug 6;14(15):5528. doi: 10.3390/jcm14155528. J Clin Med. 2025. PMID: 40807149 Free PMC article. Review.
-
Resolution of Thyroid Acropachy in a Patient Treated With Teprotumumab: A Case Report and Review of Mechanisms.Case Rep Endocrinol. 2025 Jul 18;2025:5544869. doi: 10.1155/crie/5544869. eCollection 2025. Case Rep Endocrinol. 2025. PMID: 40718626 Free PMC article.
-
Efficacy of teprotumumab therapy in patients with long-duration thyroid eye disease.Curr Opin Ophthalmol. 2023 Nov 1;34(6):487-492. doi: 10.1097/ICU.0000000000000997. Epub 2023 Aug 23. Curr Opin Ophthalmol. 2023. PMID: 37610428 Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources