A Framework for Corticomuscle Control Studies Using a Serious Gaming Approach
- PMID: 40700312
- PMCID: PMC12286066
- DOI: 10.3390/mps8040074
A Framework for Corticomuscle Control Studies Using a Serious Gaming Approach
Abstract
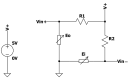
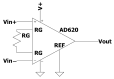
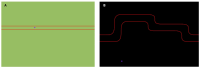
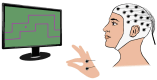
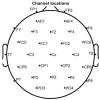
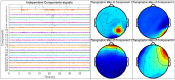
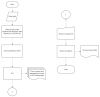
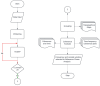
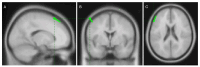
Sophisticated voluntary movements are essential for everyday functioning, making the study of how the brain controls muscle activity a central challenge in neuroscience. Investigating corticomuscular control through non-invasive electrophysiological recordings is particularly complex due to the intricate nature of neuronal signals. To address this challenge, we present a novel experimental methodology designed to study corticomuscular control using electroencephalography (EEG) and electromyography (EMG). Our approach integrates a serious gaming biofeedback system with a specialized experimental protocol for simultaneous EEG-EMG data acquisition, optimized for corticomuscular studies. This work introduces, for the first time, a method for assessing brain-muscle functional connectivity during the execution of a demanding motor task. By identifying neuronal sources linked to muscular activity, this methodology has the potential to advance our understanding of motor control mechanisms. These insights could contribute to improving clinical practices and fostering the development of novel brain-computer interface technologies.
Keywords: EEG; EMG; cortico-muscle communication; corticomuscular control; phase synchrony; reference phase analysis; serious gaming.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
The Lived Experience of Autistic Adults in Employment: A Systematic Search and Synthesis.Autism Adulthood. 2024 Dec 2;6(4):495-509. doi: 10.1089/aut.2022.0114. eCollection 2024 Dec. Autism Adulthood. 2024. PMID: 40018061 Review.
-
"In a State of Flow": A Qualitative Examination of Autistic Adults' Phenomenological Experiences of Task Immersion.Autism Adulthood. 2024 Sep 16;6(3):362-373. doi: 10.1089/aut.2023.0032. eCollection 2024 Sep. Autism Adulthood. 2024. PMID: 39371355
-
An investigation of multimodal EMG-EEG fusion strategies for upper-limb gesture classification.J Neural Eng. 2025 Jul 10;22(4). doi: 10.1088/1741-2552/ade1f9. J Neural Eng. 2025. PMID: 40480249
-
Combined Use of EMG and EEG Techniques for Neuromotor Assessment in Rehabilitative Applications: A Systematic Review.Sensors (Basel). 2021 Oct 22;21(21):7014. doi: 10.3390/s21217014. Sensors (Basel). 2021. PMID: 34770320 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

