Development and Evaluation of a Nanoparticle-Based Immunoassay for Rotavirus Detection: A Suitable Alternative to ELISA and PCR in Low-Income Setting
- PMID: 40700319
- PMCID: PMC12286212
- DOI: 10.3390/mps8040081
Development and Evaluation of a Nanoparticle-Based Immunoassay for Rotavirus Detection: A Suitable Alternative to ELISA and PCR in Low-Income Setting
Abstract
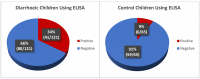
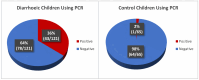
Every year, diarrhoea is responsible for >1 million deaths in children with ages from 0 to 5 years, with rotavirus as the leading cause. The regions most affected lack routine rotavirus diagnosis due to high cost, lack of necessary equipment and shortage of trained-personnel for Enzyme-Link-Immunosorbent-Assay (ELISA) and molecular methods. We report the development and evaluation of a cheap, nanoparticle-based immunoassay for routine machine-free rotavirus diagnosis. In this work, optimal conditions for oxidation of cotton swabs and aldehyde production for kit development was confirmed by Fourier-Transform Infrared Spectroscopy (FTIR). Lactoferrin (LF) needed to bind the virus to the cotton swab was immobilised on activated cotton swabs, followed by the capture of commercial rotavirus antigen on LF-immobilised swabs. This was dipped in coloured nanobeads covalently coupled to rotavirus-group-specific monoclonal antibody for visual rotavirus detection. Subsequently, rotavirus detection by nanoassay, commercial ELISA and quantitative reverse transcription PCR were compared using same set of 186 stool samples and subjected to statistical analyses. Optimal oxidisation condition was observed using 48 mg/mL NaIO4 in 0.1 M sodium acetate buffer at 35 °C for 9 h. Rotavirus detection was confirmed visually by blue colour retention on swabs after several washings. Sensitivity, specificity, positive-predictive-value and negative-predictive-value of ELISA in rotavirus detection were 60%, 84%, 53% and 88%, respectively, while our immunoassay showed performance at 88%, 94%, 82% and 96%. This immunoassay will provide effective rotavirus public health interventions in low-and-middle-income countries with high morbidity/mortality.
Keywords: ELISA; PCR; children; diagnosis; diarrhoea; immunoassay development; rotavirus.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





References
-
- GBD 2021 Diarrhoeal Diseases Collaborators Global, regional, and national age-sex-specific burden of diarrhoeal diseases, their risk factors, and aetiologies, 1990–2021, for 204 countries and territories: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Infect. Dis. 2025;25:519–536. doi: 10.1016/S1473-3099(24)00691-1. - DOI - PMC - PubMed
-
- Black R.E., Perin J., Yeung D., Rajeev T., Miller J., Elwood S.E., Platts-Mills J.A. Estimated global and regional causes of deaths from diarrhoea in children younger than 5 years during 2000-21: A systematic review and Bayesian multinomial analysis. Lancet Glob. Health. 2024;12:e919–e928. doi: 10.1016/S2214-109X(24)00078-0. - DOI - PMC - PubMed
-
- Peter A.K., Umar U. Combating diarrhoea in Nigeria: The way forward. J. Microbiol. Exp. 2018;6:191–197. doi: 10.15406/jmen.2018.06.00213. - DOI
LinkOut - more resources
Full Text Sources

