Enzymatic characterization and polyurethane biodegradation assay of two novel esterases isolated from a polluted river
- PMID: 40700380
- PMCID: PMC12286390
- DOI: 10.1371/journal.pone.0327637
Enzymatic characterization and polyurethane biodegradation assay of two novel esterases isolated from a polluted river
Abstract
The environmental ubiquity of plastic materials generates global concern, pollution, and health problems. Microorganisms and enzymes with plastic biodegradation potential are considered as environmentally friendly alternatives to address these issues. Interestingly, polluted environments exert selective pressure on native microbial communities that have the metabolic capacity to tolerate and transform different contaminants, including plastics. A number of enzymes have been described as polyurethane degraders. However, some of them do not possess complete characterization or efficient degradation rates. Hence, there is still a need to identify and characterize efficient enzymes for application in green processes for plastic recycling. Here, we used an environmental DNA sample isolated from the sediments of a polluted river in Mexico (Apatlaco River), which was used to construct a metagenomic fosmid library to explore the metabolic potential of microbial communities for polyurethane biodegradation. Functional screenings were performed on agar media containing the polyester polyurethane Impranil DLN (Impranil), and positively selected fosmid DNA was identified and sequenced by Illumina. Bioinformatic analyses identified two Acinetobacter genes (epux1 and epux2) encoding alpha/beta hydrolases. The genes were heterologously expressed to determine the capacity of their encoded proteins for Impranil clearing. Both Epux1 and Epux2 enzymes exhibited Impranil cleavage at 30 °C and 15 °C and ester group modifications were validated by infrared spectroscopy. Furthermore, the release of building blocks of the polymer was determined by GC-MS analysis, thus indicating their esterase/polyurethanase activity. Overall, our results demonstrate the potential of these novel bacterial enzymes for the hydrolysis of polyurethane with potential applications in the circular plastics economy.
Copyright: © 2025 Soto-Hernandez et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


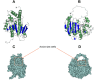






References
-
- Geyer R. Production, use, and fate of synthetic polymers. In: Letcher TM, ed. Plastic Waste and Recycling. Elsevier; 2020: 13–32.
-
- Wei R, Tiso T, Bertling J, O’Connor K, Blank LM, Bornscheuer UT. Possibilities and limitations of biotechnological plastic degradation and recycling. Nat Catal. 2020;3:867–71.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

