A role for Myo-II zipper and spaghetti squash in Gliotactin-dependent Drosophila melanogaster wing hair planar cell polarity
- PMID: 40700419
- PMCID: PMC12286378
- DOI: 10.1371/journal.pone.0328970
A role for Myo-II zipper and spaghetti squash in Gliotactin-dependent Drosophila melanogaster wing hair planar cell polarity
Abstract
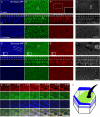
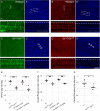
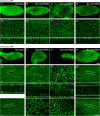
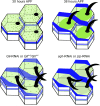
Planar cell polarity, polarization in the plane of an epithelium, is critical for tissue development. The Drosophila melanogaster wing epithelium is an important model system for planar cell polarity establishment and has greatly informed studies in vertebrates. The well-studied Frizzled-dependent and Fat - Dachsous - Four-jointed pathways establish proximal-to-distal polarity of wing hairs, while a Frizzled-independent mechanism mediated by septate junction proteins Gliotactin, Coracle, and Varicose is required for parallel alignment of neighboring hairs. In this study, we explore a requirement for the non-muscle myosin II proteins Spaghetti Squash and Zipper in wing hair planar cell polarity. We confirm a previously recognized role in hair initiation and demonstrate a second, novel Gliotactin-interacting requirement for spaghetti squash and zipper in parallel alignment. Immunolabeling experiments demonstrate that Spaghetti Squash and Zipper localize to the base of the developing hairs during the same time frame that septate junction proteins transiently relocalize to the apical cell surface. This localization is abrogated in Gliotactin loss-of-function genotypes. We propose that Gliotactin promotes Spaghetti Squash and Zipper accumulation at the cell apical surface during wing hair extension and that this apical Myosin-II complex stabilizes the developing hair base, maintaining parallel alignment of neighboring wing hairs.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Transient apical polarization of Gliotactin and Coracle is required for parallel alignment of wing hairs in Drosophila.Dev Biol. 2004 Nov 15;275(2):301-14. doi: 10.1016/j.ydbio.2004.07.040. Dev Biol. 2004. PMID: 15501220
-
Adherens junctions limit septate junction length in Drosophila midgut enterocytes but are not required for polarity.J Cell Sci. 2025 Jul 1;138(13):jcs263644. doi: 10.1242/jcs.263644. Epub 2025 Jul 10. J Cell Sci. 2025. PMID: 40485276 Free PMC article.
-
A switch to non-proliferative growth sustains Drosophila wing development during the early pupal stage.Curr Biol. 2025 Aug 18;35(16):4043-4049.e3. doi: 10.1016/j.cub.2025.07.020. Epub 2025 Aug 1. Curr Biol. 2025. PMID: 40752488
-
Shroom3 facilitates optic fissure closure via tissue alignment and reestablishment of apical-basal polarity during epithelial fusion.Dev Biol. 2025 Jun;522:91-105. doi: 10.1016/j.ydbio.2025.03.008. Epub 2025 Mar 18. Dev Biol. 2025. PMID: 40113025 Free PMC article. Review.
-
Active body surface warming systems for preventing complications caused by inadvertent perioperative hypothermia in adults.Cochrane Database Syst Rev. 2016 Apr 21;4(4):CD009016. doi: 10.1002/14651858.CD009016.pub2. Cochrane Database Syst Rev. 2016. PMID: 27098439 Free PMC article.
References
-
- Hirota Y, Meunier A, Huang S, Shimozawa T, Yamada O, Kida YS. Planar polarity of multiciliated ependymal cells involves the anterior migration of basal bodies regulated by non-muscle myosin II. Development. 2010;137(18):3037–46. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

