A functional interleukin-4 homolog is encoded in the genome of infectious laryngotracheitis virus: Unveiling a novel virulence factor
- PMID: 40700459
- PMCID: PMC12327624
- DOI: 10.1371/journal.ppat.1013219
A functional interleukin-4 homolog is encoded in the genome of infectious laryngotracheitis virus: Unveiling a novel virulence factor
Erratum in
-
Correction: A functional interleukin-4 homolog is encoded in the genome of infectious laryngotracheitis virus: Unveiling a novel virulence factor.PLoS Pathog. 2025 Dec 19;21(12):e1013791. doi: 10.1371/journal.ppat.1013791. eCollection 2025 Dec. PLoS Pathog. 2025. PMID: 41417697 Free PMC article.
Abstract
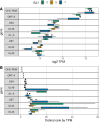
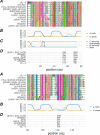
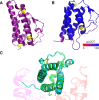
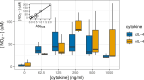
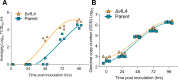
Herpesviruses have evolved numerous immune evasion tactics, persisting within their hosts through self-perpetuating strategies. One such tactic involves acquiring functional copies of host genes encoding cytokines such as IL-6 (HHV-8), IL-10 (HHV-4, HHV-5), and IL-17 (SaHV-2). These viral mimics, or virokines, can bind to cellular receptors, modulating the natural cytokine signaling to manipulate the immune response in favor of the virus or stimulate target cell growth to enhance virus replication. In the course of full-length cDNA sequencing of infectious laryngotracheitis virus (ILTV) transcripts, a previously unknown highly spliced gene was discovered in the viral genome predicted to encode a 147 amino acid protein with similarity to vertebrate interleukin-4. The three-intron gene structure was precisely conserved with chicken and other vertebrate IL-4 homologs, and the amino acid sequence displayed structural conservation with vertebrate homologs at the primary, secondary, and tertiary levels based on computational modeling. The viral IL-4 gene was subsequently identified in all sequenced ILTV genomes. The mature transcript was highly expressed both in vitro and in vivo, and protein expression in infected cells was confirmed using LC-MS/MS. Phylogenetic analyses, along with the conserved gene structure, suggested direct capture from a Galliformes host. Functionally, an LPS-stimulation assay showed that the expressed viral IL-4 homolog stimulated nitric oxide production in a macrophage cell line at comparable levels to recombinant chicken IL-4. A recombinant virus lacking vIL-4 exhibited slightly higher titers in cell culture compared to the parental strain. In vivo bird studies demonstrated reduced pathogenicity of the vIL-4 knockout compared to wildtype. These results represent the first report of a previously unknown virokine encoded in the ILTV genome expressing a functional IL-4 homolog and virulence factor.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Garcia M, Spatz S, Guy JS. Infectious laryngotracheitis. In: Swayne DE, Glisson JR, McDougald LR, Nolan LK, Suarez DL, Nair V, editors. Diseases of poultry. Ames, IA: Wiley-Blackwell; 2013.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

