Targeting tumor-associated CCR2+ macrophages to inhibit pancreatic cancer recurrence following irreversible electroporation
- PMID: 40700478
- PMCID: PMC12285706
- DOI: 10.1126/sciadv.adw2937
Targeting tumor-associated CCR2+ macrophages to inhibit pancreatic cancer recurrence following irreversible electroporation
Abstract
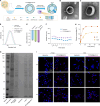
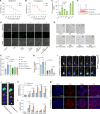
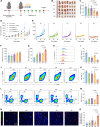
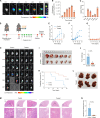
Pancreatic ductal adenocarcinoma (PDAC) is a highly lethal malignancy with pronounced resistance to conventional therapies. Irreversible electroporation (IRE) is a promising therapy for PDAC; however, its clinical efficacy is limited by a high recurrence rate. Here, using a preclinical PDAC model, we characterized the tumor immune microenvironment following insufficient IRE (iIRE) through single-cell RNA sequencing. We found that iIRE induces a CCR2+ tumor-associated macrophage (CCR2+ TAM)-mediated immunosuppressive microenvironment in residual tumors. Consequently, we developed a macrophage-based proteolipid vesicle (mPLV) coencapsulating the CCR2 antagonist PF-4136309 (PF) and gemcitabine (GEM), named PF/GEM@mPLV. Our findings suggest that PF/GEM@mPLV achieves high drug accumulation within tumors through iIRE-induced inflammation. Reduction of CCR2+ TAMs enhances antitumor immunity and improves chemotherapeutic response. PF/GEM@mPLV markedly inhibits tumor recurrence following iIRE, diminishes hepatic metastases, and prolongs survival in preclinical PDAC models. These findings uncover the role of CCR2+ TAMs in iIRE-induced immunosuppression, offering a promising strategy to enhance the clinical potential of IRE in PDAC.
Figures



References
-
- Bray F., Laversanne M., Sung H. Y. A., Ferlay J., Siegel R. L., Soerjomataram I., Jemal A., Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263 (2024). - PubMed
-
- Tempero M. A., Malafa M. P., Al-Hawary M., Behrman S. W., Benson A. B., Cardin D. B., Chiorean E. G., Chung V., Czito B., Del Chiaro M., Dillhoff M., Donahue T. R., Dotan E., Ferrone C. R., Fountzilas C., Hardacre J., Hawkins W. G., Klute K., Ko A. H., Kunstman J. W., LoConte N., Lowy A. M., Moravek C., Nakakura E. K., Narang A. K., Obando J., Polanco P. M., Reddy S., Reyngold M., Scaife C., Shen J., Vollmer C., Wolff R. A., Wolpin B. M., Lynn B., George G. V., Pancreatic adenocarcinoma, version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 19, 439–457 (2021). - PubMed
-
- Grossberg A. J., Chu L. C., Deig C. R., Fishman E. K., Hwang W. L., Maitra A., Marks D. L., Mehta A., Nabavizadeh N., Simeone D. M., Weekes C. D., Thomas C. R. Jr., Multidisciplinary standards of care and recent progress in pancreatic ductal adenocarcinoma. CA Cancer J. Clin. 70, 375–403 (2020). - PMC - PubMed
-
- Suker M., Beumer B. R., Sadot E., Marthey L., Faris J. E., Mellon E. A., El-Rayes B. F., Wang-Gillam A., Lacy J., Hosein P. J., Moorcraft S. Y., Conroy T., Hohla F., Allen P., Taieb J., Hong T. S., Shridhar R., Chau I., van Eijck C. H., Koerkamp B. G., FOLFIRINOX for locally advanced pancreatic cancer: A systematic review and patient-level meta-analysis. Lancet Oncol. 17, 801–810 (2016). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

