Selective engagement of prefrontal VIP neurons in reversal learning
- PMID: 40700480
- PMCID: PMC12285700
- DOI: 10.1126/sciadv.adt4945
Selective engagement of prefrontal VIP neurons in reversal learning
Abstract
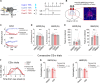
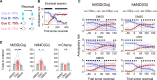
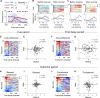
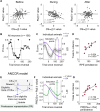
To gain insights into neural mechanisms enabling behavioral adaptations to complex and multidimensional environmental dynamics, we examined roles of vasoactive intestinal polypeptide (VIP)-expressing neurons in mouse medial prefrontal cortex (mPFC) in probabilistic reversal learning. Behaviorally, manipulating VIP neuronal activity left probabilistic classical conditioning unaffected but severely impaired reversal learning. Physiologically, conditioned cue-associated VIP neuronal responses changed abruptly after encountering an unexpected reward. They also conveyed strong reward prediction error signals during behavioral reversal, but not before or after, unlike pyramidal neurons that consistently conveyed error signals throughout all phases. Furthermore, the signal's persistence across trials correlated with reversal learning duration. These results suggest that mPFC VIP neurons play crucial roles in rapid reversal learning, but not in gradual value updating under stable probabilistic contingencies, by monitoring salient deviations from ongoing environmental contingencies and imposing error-correction signals during behavioral adjustments. These findings shed light on the intricate cortical circuit dynamics underpinning behavioral flexibility in complex, multifaceted environments.
Figures


Similar articles
-
Peripuberty Is a Sensitive Period for Prefrontal Parvalbumin Interneuron Activity to Impact Adult Cognitive Flexibility.Dev Neurosci. 2025;47(2):127-138. doi: 10.1159/000539584. Epub 2024 Jun 3. Dev Neurosci. 2025. PMID: 38830346 Free PMC article.
-
Flexible decision-making is related to strategy learning, vicarious trial and error, and medial prefrontal rhythms during spatial set-shifting.Learn Mem. 2024 Jul 22;31(7):a053911. doi: 10.1101/lm.053911.123. Print 2024 Jul. Learn Mem. 2024. PMID: 39038921 Free PMC article.
-
Enhanced cognitive flexibility and phasic striatal dopamine dynamics in a mouse model of low striatal tonic dopamine.Neuropsychopharmacology. 2024 Sep;49(10):1600-1608. doi: 10.1038/s41386-024-01868-5. Epub 2024 May 2. Neuropsychopharmacology. 2024. PMID: 38698264
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
-
Education support services for improving school engagement and academic performance of children and adolescents with a chronic health condition.Cochrane Database Syst Rev. 2023 Feb 8;2(2):CD011538. doi: 10.1002/14651858.CD011538.pub2. Cochrane Database Syst Rev. 2023. PMID: 36752365 Free PMC article.
References
-
- McDonald R. J., White N. M., A triple dissociation of memory systems: Hippocampus, amygdala, and dorsal striatum. Behav. Neurosci. 107, 3–22 (1993). - PubMed
-
- Kim J. J., Baxter M. G., Multiple brain-memory systems: The whole does not equal the sum of its parts. Trends Neurosci. 24, 324–330 (2001). - PubMed
-
- Packard M. G., Knowlton B. J., Learning and memory functions of the basal ganglia. Annu. Rev. Neurosci. 25, 563–593 (2002). - PubMed
-
- Gold P. E., Coordination of multiple memory systems. Neurobiol. Learn. Mem. 82, 230–242 (2004). - PubMed
-
- Squire L. R., Memory systems of the brain: A brief history and current perspective. Neurobiol. Learn. Mem. 82, 171–177 (2004). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

