Metabotissugenic citrate biomaterials orchestrate bone regeneration via citrate-mediated signaling pathways
- PMID: 40700481
- PMCID: PMC12285708
- DOI: 10.1126/sciadv.ady2862
Metabotissugenic citrate biomaterials orchestrate bone regeneration via citrate-mediated signaling pathways
Abstract
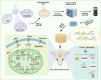
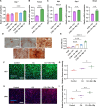
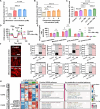
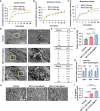
Bone regeneration requires coordinated anabolic and catabolic signaling, yet the interplay between mammalian target of rapamycin complex 1 (mTORC1) and adenosine monophosphate-activated protein kinase (AMPK) pathways remains unclear. This study reveals that citrate, glutamine, and magnesium synergistically activate both pathways via calcium/calmodulin-dependent protein kinase kinase 2 (CaMKK2)- and protein kinase B (Akt)-dependent signaling, bypassing the traditional adenosine monophosphate (AMP)/adenosine triphosphate (ATP) sensing mechanism. This dual activation supports sustained energy metabolism during osteogenesis and challenges the canonical antagonism between mTORC1 and AMPK. We developed CitraBoneQMg, a citrate-based biomaterial incorporating these components via one-pot synthesis. CitraBoneQMg provides sustained release, photoluminescent and photoacoustic imaging capabilities, and tunable mechanical properties. In vitro, it promotes osteogenesis by enhancing alkaline phosphatase (ALP) activity, osteogenic gene expression, and calcium deposition. In vivo, it accelerates bone regeneration in a rat calvarial defect model while promoting anti-inflammatory and neuroregenerative responses. We define this integrated effect as "metabotissugenesis," offering a metabolically optimized approach to orthopedic biomaterial design.
Figures





References
-
- Qiao W., Wong K. H. M., Shen J., Wang W., Wu J., Li J., Lin Z., Chen Z., Matinlinna J. P., Zheng Y., Wu S., Liu X., Lai K. P., Chen Z., Lam Y. W., Cheung K. M. C., Yeung K. W. K., TRPM7 kinase-mediated immunomodulation in macrophage plays a central role in magnesium ion-induced bone regeneration. Nat. Commun. 12, 2885 (2021). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

