Engineering hybrid nanoparticles for targeted codelivery of triptolide and CYP3A4-siRNA against pulmonary metastatic melanoma
- PMID: 40700483
- PMCID: PMC12285696
- DOI: 10.1126/sciadv.adv6990
Engineering hybrid nanoparticles for targeted codelivery of triptolide and CYP3A4-siRNA against pulmonary metastatic melanoma
Abstract
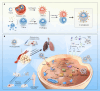
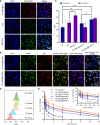
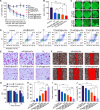
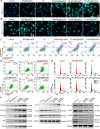
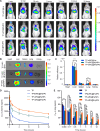
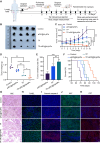
Pulmonary metastatic melanoma (PMM) is an aggressive malignancy with limited response and rapid resistance to clinical chemotherapy, radiotherapy, immunotherapy, and biological therapies. Here, we developed a targeted biomimetic drug delivery system, TP-siRC@tHyNPs, by fusing exosomes derived from engineered cells overexpressing DR5 single-chain variable fragments (DR5-Exo) with liposomes coencapsulating triptolide (TP) and CYP3A4-siRNA (TP-siRC@Lip). DR5-Exo facilitated the targeted delivery of drug to tumor cells through DR5 receptor recognition and simultaneously activated apoptotic pathways. Moreover, CYP3A4-siRNA effectively prolonged the half-life of TP, thereby enhancing its antiproliferative and pro-apoptotic effects. Mechanistic studies revealed that TP-siRC@tHyNPs induced immunogenic cell death, reprogrammed macrophage polarization, arrested cell cycle progression, and triggered apoptotic pathways. In vivo experiments demonstrated that TP-siRC@tHyNPs specifically accumulated in lung tissue, notably inhibiting the growth of PMM while exhibiting negligible toxicity in tumor-bearing mice. Overall, this study provides a promising strategy for targeting PMM treatment, improving therapeutic efficacy while reducing off-target toxicity.
Figures



Similar articles
-
A Novel Triptolide Nano-Liposome with Mitochondrial Targeting for Treatment of Hepatocellular Carcinoma.Int J Nanomedicine. 2024 Dec 3;19:12975-12998. doi: 10.2147/IJN.S498099. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39654802 Free PMC article.
-
A Nanoplatform Targeting Cascade Pathways for Enhanced Triptolide Delivery in Acute Kidney Injury Therapy.Adv Healthc Mater. 2025 Jun;14(16):e2500595. doi: 10.1002/adhm.202500595. Epub 2025 May 12. Adv Healthc Mater. 2025. PMID: 40350983
-
Dendritic Polylysine with Paclitaxel and Triptolide Codelivery for Enhanced Cancer Ferroptosis through the Accumulation of ROS.ACS Appl Mater Interfaces. 2024 Apr 24;16(16):20143-20156. doi: 10.1021/acsami.4c00558. Epub 2024 Apr 10. ACS Appl Mater Interfaces. 2024. PMID: 38597227
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Advances in siRNA-loaded liposomes for lung cancer: Challenges and future perspectives.Int J Biol Macromol. 2025 Aug;319(Pt 1):145461. doi: 10.1016/j.ijbiomac.2025.145461. Epub 2025 Jun 21. Int J Biol Macromol. 2025. PMID: 40550440 Review.
Cited by
-
Preparation and Characterization of Cyperus-Derived Exosomes Loaded with Selenium Nanoparticles for Selenium Delivery Based on Exosome Protein Quantitation.Foods. 2025 Aug 4;14(15):2724. doi: 10.3390/foods14152724. Foods. 2025. PMID: 40807660 Free PMC article.
References
-
- Long G. V., Swetter S. M., Menzies A. M., Gershenwald J. E., Scolyer R. A., Cutaneous melanoma. Lancet 402, 485–502 (2023). - PubMed
-
- Skudalski L., Waldman R., Kerr P. E., Grant-Kels J. M., Melanoma: An update on systemic therapies. J. Am. Acad. Dermatol. 86, 515–524 (2022). - PubMed
-
- Rashid S., Shaughnessy M., Tsao H., Melanoma classification and management in the era of molecular medicine. Dermatol. Clin. 41, 49–63 (2023). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

