Genetic foundations of interindividual neurophysiological variability
- PMID: 40700495
- PMCID: PMC12285703
- DOI: 10.1126/sciadv.ads7544
Genetic foundations of interindividual neurophysiological variability
Abstract
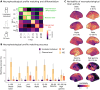
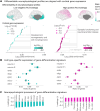
Neurophysiological brain activity shapes cognitive functions and individual traits. Here, we investigated the extent to which individual neurophysiological properties are genetically determined and how these adult traits align with cortical gene expression patterns across development. Using task-free magnetoencephalography in monozygotic and dizygotic twins, as well as unrelated individuals, we found that neurophysiological traits were significantly more similar between monozygotic twins, indicating a genetic influence, although individual-specific variability remained predominant. These heritable brain dynamics were mainly associated with genes involved in neurotransmission, expressed along a topographical gradient that mirrors psychological functions, including attention, planning, and emotional processes. Furthermore, the cortical expression patterns of genes associated with individual differentiation aligned most strongly with gene expression profiles observed during adulthood in previously published longitudinal datasets. These findings underscore a persistent genetic influence on neurophysiological activity, supporting individual cognitive and behavioral variability.
Figures


Update of
-
Genetic Foundations of Inter-individual Neurophysiological Variability.bioRxiv [Preprint]. 2025 Apr 29:2024.07.19.604292. doi: 10.1101/2024.07.19.604292. bioRxiv. 2025. Update in: Sci Adv. 2025 Jul 25;11(30):eads7544. doi: 10.1126/sciadv.ads7544. PMID: 39071281 Free PMC article. Updated. Preprint.
References
-
- Kaufmann T., Alnæs D., Doan N. T., Brandt C. L., Andreassen O. A., Westlye L. T., Delayed stabilization and individualization in connectome development are related to psychiatric disorders. Nat. Neurosci. 20, 513–515 (2017). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

