Dynamic change of calcium-rich compartments during coccolithophore biomineralization
- PMID: 40700501
- PMCID: PMC12285720
- DOI: 10.1126/sciadv.adv0618
Dynamic change of calcium-rich compartments during coccolithophore biomineralization
Abstract
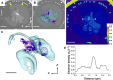
Coccolithophores are abundant marine phytoplankton that produce biomineralized calcite scales, called coccoliths, which sequester substantial amounts of carbon and play a substantial role in biogeochemical cycles. However, mechanisms underlying the storage and transport of ions essential for calcification remain unresolved. We used ptychographic x-ray computed tomography under cryogenic conditions to visualize intracellular calcium-rich structures involved in the storage of calcium ions in the coccolithophore species Chrysotila carterae. During calcification, we observed a range of structures, from small electron-dense bodies within larger compartments to denser and distributed globular compartments, before returning to small bodies once scale formation is complete. Nanobeam-scanning x-ray fluorescence measurements further revealed that these electron-dense bodies are rich in phosphorus and calcium (molar ratio of ~4:1). The dynamic nature of structures suggests that these bodies are part of the required cellular calcium ion transport pathways, a fundamental process critical for understanding the response of coccolithophores to climate change.
Figures






Similar articles
-
Exploring proteins within the coccolith matrix.Sci Rep. 2024 Dec 30;14(1):31821. doi: 10.1038/s41598-024-83052-9. Sci Rep. 2024. PMID: 39738514 Free PMC article.
-
Coccolithophore biomineralization: New questions, new answers.Semin Cell Dev Biol. 2015 Oct;46:11-6. doi: 10.1016/j.semcdb.2015.10.027. Epub 2015 Oct 20. Semin Cell Dev Biol. 2015. PMID: 26498037 Review.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Native-state imaging of calcifying and noncalcifying microalgae reveals similarities in their calcium storage organelles.Proc Natl Acad Sci U S A. 2018 Oct 23;115(43):11000-11005. doi: 10.1073/pnas.1804139115. Epub 2018 Oct 4. Proc Natl Acad Sci U S A. 2018. PMID: 30287487 Free PMC article.
-
Coccolithophore calcification: Changing paradigms in changing oceans.Acta Biomater. 2021 Jan 15;120:4-11. doi: 10.1016/j.actbio.2020.07.050. Epub 2020 Aug 4. Acta Biomater. 2021. PMID: 32763469 Review.
References
-
- Ziveri P., de Bernardi B., Baumann K. H., Stoll H. M., Mortyn P. G., Sinking of coccolith carbonate and potential contribution to organic carbon ballasting in the deep ocean. Deep Sea Res. Pt. II 54, 659–675 (2007).
-
- Milliman J. D., Droxler A. W., Neritic and pelagic carbonate sedimentation in the marine environment: Ignorance is not bliss. Geol. Rundsch. 85, 496–504 (1996).
-
- Ridgwell A., Zeebe R. E., The role of the global carbonate cycle in the regulation and evolution of the Earth system. Earth Planet. Sci. Lett. 234, 299–315 (2005).
-
- Van der Wal P., Dejong E. W., Westbroek P., Debruijn W. C., Mulderstapel A. A., Polysaccharide localization, coccolith formation, and golgi dynamics in the coccolithophorid Hymenomonas carterae. J. Ultrastruct. Res. 85, 139–158 (1983). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

