M6A-dependent RNA condensation underlies FUS autoregulation and can be harnessed for ALS therapy development
- PMID: 40700505
- PMCID: PMC12285722
- DOI: 10.1126/sciadv.adx1357
M6A-dependent RNA condensation underlies FUS autoregulation and can be harnessed for ALS therapy development
Abstract
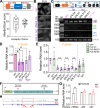
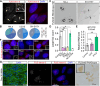
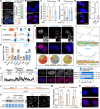
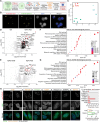
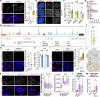
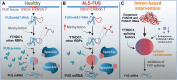
Mutations in the FUS gene cause aggressive amyotrophic lateral sclerosis (ALS-FUS). Beyond mRNA, FUS generates partially processed transcripts retaining introns 6 and 7. We demonstrate that these FUSint6&7-RNA molecules form nuclear condensates, scaffolded by the highly structured intron 7 and associated with nuclear speckles. Using hybridization-proximity labeling proteomics, we show that the FUSint6&7-RNA condensates are enriched for splicing factors and the N6-methyladenosine (m6A) reader YTHDC1. These ribonucleoprotein structures facilitate posttranscriptional FUS splicing and depend on m6A/YTHDC1 for integrity. In cells expressing mutant FUS, FUSint6&7-RNAs become hypermethylated, which in turn stimulates their condensation and splicing. We further show that FUS protein is repelled by m6A. Thus, ALS-FUS mutations may cause abnormal activation of FUS posttranscriptional splicing through altered RNA methylation. Notably, ectopic expression of FUS intron 7 sequences dissolves endogenous FUSint6&7-RNA condensates, down-regulating FUS mRNA and protein. Our findings reveal a condensation-dependent mechanism regulating FUS splicing, with possible therapeutic implications for ALS.
Figures


References
-
- Ratti A., Buratti E., Physiological functions and pathobiology of TDP-43 and FUS/TLS proteins. J. Neurochem. 138, 95–111 (2016). - PubMed
-
- Kwiatkowski T. J. Jr., Bosco D. A., Leclerc A. L., Tamrazian E., Vanderburg C. R., Russ C., Davis A., Gilchrist J., Kasarskis E. J., Munsat T., Valdmanis P., Rouleau G. A., Hosler B. A., Cortelli P., de Jong P. J., Yoshinaga Y., Haines J. L., Pericak-Vance M. A., Yan J., Ticozzi N., Siddique T., McKenna-Yasek D., Sapp P. C., Horvitz H. R., Landers J. E., Brown R. H. Jr., Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 323, 1205–1208 (2009). - PubMed
-
- Vance C., Rogelj B., Hortobagyi T., De Vos K. J., Nishimura A. L., Sreedharan J., Hu X., Smith B., Ruddy D., Wright P., Ganesalingam J., Williams K. L., Tripathi V., Al-Saraj S., Al-Chalabi A., Leigh P. N., Blair I. P., Nicholson G., de Belleroche J., Gallo J. M., Miller C. C., Shaw C. E., Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 323, 1208–1211 (2009). - PMC - PubMed
-
- Moens T. G., Da Cruz S., Neumann M., Shelkovnikova T. A., Shneider N. A., Van Den Bosch L., Amyotrophic lateral sclerosis caused by FUS mutations: Advances with broad implications. Lancet Neurol. 24, 166–178 (2025). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

