Protective envelope dimer epitope-like antibodies are elicited against dengue virus in children after infection and vaccination
- PMID: 40700517
- PMCID: PMC12351287
- DOI: 10.1126/scitranslmed.adq0571
Protective envelope dimer epitope-like antibodies are elicited against dengue virus in children after infection and vaccination
Abstract
Cross-reactive antibodies to epitopes that span envelope proteins on the virion surface are hypothesized to protect against dengue virus (DENV) infection and disease. Here, we measured antibodies targeting a quaternary epitope called the envelope dimer epitope (EDE) as well as neutralizing and binding antibodies and evaluated their association with DENV infection, vaccine response, and disease outcome in dengue-vaccinated (n = 164) and dengue-unvaccinated children (n = 88) within a longitudinal cohort in Cebu, Philippines (n = 2996). Antibodies targeting EDE were prevalent and associated with broad neutralization of mature DENV1 to DENV4 virions in those with evidence of at least two prior DENV infections but were mostly absent in those with only one prior infection. Subsequent infection and vaccination boosted titers of EDE-like antibodies, neutralizing antibodies, and DENV-binding antibodies. EDE-like antibodies were associated with reduced risk of symptomatic dengue and more severe dengue and statistically explained the protective effect of binding and neutralizing antibodies on dengue. Thus, antibodies targeting quaternary epitopes help explain the broad cross-protection observed in those with multiple prior DENV exposures, making them useful for evaluation and development of future vaccines and therapeutics.
Conflict of interest statement
Competing interests
MY, MVC, JVD, KAA, AMC, and AKS report receiving salaries from 2017 onwards as part of an ongoing separate study (effectiveness of the tetravalent dengue vaccine, CYD-TDV [Dengvaxia] in the Philippines) sponsored by the University of the Philippines Manila and funded by Sanofi Pasteur. JD was an unpaid external consultant in the Extended Study Group for dengue vaccine effectiveness evaluation studies in Asia in 2015 convened by Sanofi Pasteur and is an unpaid investigator of an ongoing separate study (effectiveness of the tetravalent dengue vaccine, CYD-TDV [Dengvaxia] in the Philippines) sponsored by the University of the Philippines Manila and funded by Sanofi Pasteur. AMdS is listed as an inventor on pending patent applications filed by the University of North Carolina related to flavivirus vaccines and diagnostics (CHIMERIC DENGUE VIRUS E GLYCOPROTEINS COMPRISING MUTANT DOMAIN I AND DOMAIN II HINGE REGIONS, Patent No.: US 9,821,050 B2; METHODS AND COMPOSITIONS FOR DENGUE VIRUS VACCINES, Patent No.: US 10,053,493 B2; METHODS AND COMPOSITIONS FOR RECOMBINANT DENGUE VIRUSES FOR VACCINE AND DIAGNOSTIC DEVELOPMENT, Patent No.: US 10,398,768 B2; METHODS AND COMPOSITIONS FOR DENGUE VIRUS VACCINES AND DIAGNOSTICS, Pub. Da: US 2018/028. 4,2011; METHODS AND COMPOSITIONS FOR ZIKA VIRUS VACCINES, Pub. No.: US 2019/0023745 A1; METHODS AND COMPOSITIONS FOR STABILIZED RECOMBINANT FLAVIVIRUS E PROTEIN DIMERS, International Application No.: PCT/US2020/045241). All other authors declare no competing interests.
Figures

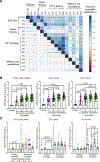

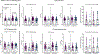

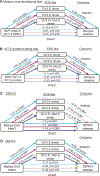
Update of
-
Envelope-dimer epitope-like broadly protective antibodies against dengue in children following natural infection and vaccination.medRxiv [Preprint]. 2024 May 1:2024.04.30.24306574. doi: 10.1101/2024.04.30.24306574. medRxiv. 2024. Update in: Sci Transl Med. 2025 Jul 23;17(808):eadq0571. doi: 10.1126/scitranslmed.adq0571. PMID: 38746253 Free PMC article. Updated. Preprint.
References
-
- Abe H et al. , Re-emergence of dengue virus serotype 3 infections in Gabon in 2016–2017, and evidence for the risk of repeated dengue virus infections. Int J Infect Dis 91, 129–136 (2020). - PubMed
-
- Cattarino L, Rodriguez-Barraquer I, Imai N, Cummings DAT, Ferguson NM, Mapping global variation in dengue transmission intensity. Sci Transl Med 12, (2020). - PubMed
-
- Sridhar S et al. , Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy. N Engl J Med 379, 327–340 (2018). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

