Perfluorooctanesulfonic acid (PFOS) antagonizes gamma-aminobutyric acid (GABA) receptors in larval zebrafish and mammalian models
- PMID: 40700607
- PMCID: PMC12560803
- DOI: 10.1093/toxsci/kfaf101
Perfluorooctanesulfonic acid (PFOS) antagonizes gamma-aminobutyric acid (GABA) receptors in larval zebrafish and mammalian models
Abstract
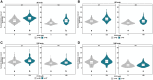
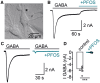
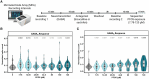
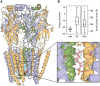
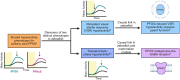
Per- and polyfluoroalkyl substances are a class of synthetic chemicals detected ubiquitously in the environment, humans, and wildlife. Perfluorooctanesulfonic acid (PFOS) is one prevalent chemical previously shown to cause adverse effects on nervous system function across in vivo and in vitro models, including dark-phase hyperactivity in larval zebrafish. The objective of this study was to evaluate the role of gamma-aminobutyric acid receptors (GABARs), GABAAR and GABABR, as mediators of dark-phase hyperactivity in PFOS-exposed larval zebrafish. Zebrafish were acutely exposed to 7.87 to 120 μM PFOS, 0.68 to 12.4 μM picrotoxin (GABAAR antagonist), 0.77 to 14.05 μM propofol (GABAAR-positive allosteric modulator), 4.4 to 80 μM saclofen (GABABR antagonist), 0.43 to 7.87 μM CGP13501 (GABABR-positive allosteric modulator), or the solvent control 0.4% dimethyl sulfoxide 60 min before behavior assessment at 5 days post fertilization. Co-exposures to positive allosteric modulators and PFOS were performed. Acute exposure to PFOS caused transient dark-phase hyperactivity. Concentration-dependent dark-phase hypoactivity was observed following acute propofol or CGP13501 exposure, in contrast to the concentration-dependent hyperactivity caused by acute picrotoxin exposure. Saclofen exposure provoked a modest reduction in dark-phase motor activity at the highest concentration tested. PFOS-induced hyperactivity was rescued to baseline activity by co-exposure to propofol or CGP13501. To assess relevance across species, electrophysiological measurements were performed in cultured mouse cortical neurons and BrainSpheres derived from human-induced pluripotent stem cells. PFOS exposure reduced GABAAR-mediated currents in mouse neurons. GABAAR- and GABABR-dependent units in BrainSphere-derived neural networks exhibited increased spiking activity following PFOS exposure. This study demonstrates that PFOS antagonizes GABARs in zebrafish, mouse, and human experimental systems. Taken together, this study supports the concept that early life-stage zebrafish can be used to rapidly identify causative mechanisms, conserved across taxa, by which xenobiotic agents alter neuroactivity.
Keywords: GABA; PFOS; behavior; neurotoxicology; zebrafish.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Society of Toxicology.
Figures




References
-
- Andrews DQ, Naidenko OV. 2020. Population-wide exposure to per- and polyfluoroalkyl substances from drinking water in the United States. Environ Sci Technol Lett. 7:931–936. 10.1021/acs.estlett.0c00713 - DOI
-
- Assad N, Luz WL, Santos-Silva M, Carvalho T, Moraes S, Picanço-Diniz DLW, Bahia CP, Oliveira Batista EDJ, Da Conceição Passos A, Oliveira KRHM, et al. 2020. Acute restraint stress evokes Anxiety-Like behavior mediated by telencephalic inactivation and GabAergic dysfunction in zebrafish brains. Sci Rep. 10:5551. 10.1038/s41598-020-62077-w - DOI - PMC - PubMed
-
- Banks RE, Smart BE, Tatlow JC, editors. 1994. Organofluorine chemistry: principles and commercial applications. Boston (MA): Springer US : Imprint : Springer.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

