Trends in Prenatal Exposure to Antiseizure Medications Over the Past Decade: A Nationwide Study
- PMID: 40700674
- PMCID: PMC12288843
- DOI: 10.1212/WNL.0000000000213933
Trends in Prenatal Exposure to Antiseizure Medications Over the Past Decade: A Nationwide Study
Abstract
Background and objectives: Prenatal exposure to certain antiseizure medications (ASMs) is associated with established or suspected risks of congenital malformations and neurodevelopmental disorders. Large-scale, real-life data are essential to guide efforts to mitigate these risks. Our objective was to assess trends in prenatal exposure to ASMs over the past decade according to medication safety profiles.
Methods: This nationwide, population-based study is based on comprehensive data of the French National Mother-Child Register EPI-MERES. All ASM-exposed pregnancies ended between 2013 and 2021 were included. ASM-exposed pregnancies' frequency and characteristics (maternal sociodemographics and morbidities, pregnancy outcome, and ASM treatment modalities) were assessed considering 3 safety categories: (1) ASMs considered the safest (lamotrigine and levetiracetam); (2) ASMs with uncertain risk, including pregabalin, gabapentin, and newer ASMs (e.g., lacosamide and zonisamide); and (3) ASMs with acknowledged risk, including valproic acid, valpromide, carbamazepine, and topiramate.
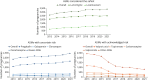
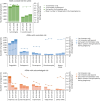
Results: Between 2013 and 2021, 55,801 pregnancies were exposed to ≥1 ASM. Pregnancies exposed to the safest ASMs increased by +30%. Meanwhile, prenatal exposure to valproic acid and valpromide dramatically decreased due to decreasing numbers of exposed pregnancies (-84% and -89%, respectively), increasing termination rate of exposed pregnancies (+23% and +28%, respectively), and among those ended in childbirth, decreasing numbers with multiple valproate dispensations (-86% and -93%, respectively) or sustained exposure throughout pregnancy (-91% and -96%, respectively). Prenatal exposure to carbamazepine and topiramate barely decreased, with almost 600 newborns still exposed to each of these ASMs in 2019-2021. Pregabalin and gabapentin became widely used during pregnancy, resulting in more and more newborns prenatally exposed (+28%), and for pregabalin increasingly with multiple dispensations (+65%) and sustained exposure throughout pregnancy (+171%). The numbers of pregnancies and newborns exposed to newer ASMs also sharply increased (+140% and +60%, respectively). Overall, prenatal exposure to ASMs with acknowledged or uncertain risk disproportionately concerned pregnant women with a low level of resources (18.5% and 17.9%, respectively, vs 13%-14% among pregnancies exposed to the safest ASMs or ASM-unexposed).
Discussion: Despite a sharp shift from valproate to safer ASMs, prenatal exposure to other ASMs with acknowledged or uncertain risks has persisted or even increased, particularly among the most socially disadvantaged populations, requiring additional risk minimization measures.
Conflict of interest statement
The authors report no relevant disclosures. Go to
Figures

References
-
- Coste J, Mandereau-Bruno L, Carcaillon-Bentata L, Mikaeloff Y, Bouilleret V. Prevalence, demographic and spatial distribution of treated epilepsy in France in 2020: a study based on the French national health data system. J Neurol. 2024;271(1):519-525. doi: 10.1007/s00415-023-11953-2 - DOI - PMC - PubMed
-
- Lai W, He S, Zhou D, Chen L. Managing reproductive problems in women with epilepsy of childbearing age. Acta Epileptol. 2021;3(1):28. doi: 10.1186/s42494-021-00062-0 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
