Automatic Abstraction of Computed Tomography Imaging Indication Using Natural Language Processing for Evaluation of Surveillance Patterns in Long-Term Lung Cancer Survivors
- PMID: 40700679
- PMCID: PMC12309515
- DOI: 10.1200/CCI-24-00279
Automatic Abstraction of Computed Tomography Imaging Indication Using Natural Language Processing for Evaluation of Surveillance Patterns in Long-Term Lung Cancer Survivors
Abstract
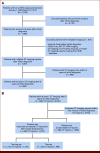
Purpose: Despite its routine use to monitor patients with lung cancer (LC), real-world evaluations of the impact of computed tomography (CT) surveillance on overall survival (OS) have been inconsistent. A major confounder is the absence of imaging indications because patients undergo CT scans for purposes beyond surveillance, like symptom evaluations (eg, cough) linked to poor survival. We propose a novel natural language processing model to predict CT imaging indications (surveillance v others).
Methods: We used electronic health records of 585 long-term LC survivors (≥5 years) at Stanford, followed for up to 22 years. Their 3,362 post-5-year CT reports (including 1,672 manually annotated) were used for modeling by integrating structured variables (eg, CT intervals) with key-phrase analysis of radiology reports. Naïve analysis compared OS in patients with CT for any indications (including symptoms) versus those without post-5-year CT, as in previous studies. Using model-predicted indications, we conducted exploratory analyses to compare OS between those with post-5-year surveillance CT and those without.
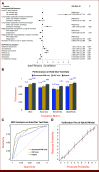
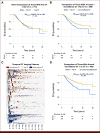
Results: The model showed high discrimination (AUC, 0.86), with key predictors including a longer interval (≥6-month) from the previous CT (odds ratios [OR], 5.50; P < .001) and surveillance-related key phrases (OR, 1.37; P = .03). Propensity-adjusted survival analysis indicated better OS for patients with any post-5-year surveillance CT versus those without (adjusted hazard ratio, 0.60; P = .016). By contrast, no significant survival difference was found (P = .53) between patients with any CT versus those without post-5-year CT.
Conclusion: Our model abstracted CT indications from real-world data with high discrimination. Exploratory analyses revealed the obscured imaging-OS association when considering indications, highlighting the model's potential for future real-world studies.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures

Similar articles
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Are Current Survival Prediction Tools Useful When Treating Subsequent Skeletal-related Events From Bone Metastases?Clin Orthop Relat Res. 2024 Sep 1;482(9):1710-1721. doi: 10.1097/CORR.0000000000003030. Epub 2024 Mar 22. Clin Orthop Relat Res. 2024. PMID: 38517402
-
Does the Presence of Missing Data Affect the Performance of the SORG Machine-learning Algorithm for Patients With Spinal Metastasis? Development of an Internet Application Algorithm.Clin Orthop Relat Res. 2024 Jan 1;482(1):143-157. doi: 10.1097/CORR.0000000000002706. Epub 2023 Jun 12. Clin Orthop Relat Res. 2024. PMID: 37306629 Free PMC article.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
Bronchoscopy-guided antimicrobial therapy for cystic fibrosis.Cochrane Database Syst Rev. 2024 May 3;5(5):CD009530. doi: 10.1002/14651858.CD009530.pub5. Cochrane Database Syst Rev. 2024. PMID: 38700027 Free PMC article.
References
-
- Miller KD, Nogueira L, Devasia T, et al. : Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin 72:409-436, 2022 - PubMed
-
- National Cancer Institute: SEER Cancer Stat Facts: Lung and Bronchus Cancer. National Cancer Institute. 2023. https://seer.cancer.gov/statfacts/html/lungb.html
-
- Johnson BE: Second lung cancers in patients after treatment for an initial lung cancer. J Natl Cancer Inst 90:1335-1345, 1998 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

