Cerebrospinal fluid lipoprotein-mediated cholesterol delivery to neurons is impaired in Alzheimer's disease and involves APOE4
- PMID: 40701521
- PMCID: PMC12391596
- DOI: 10.1016/j.jlr.2025.100865
Cerebrospinal fluid lipoprotein-mediated cholesterol delivery to neurons is impaired in Alzheimer's disease and involves APOE4
Abstract
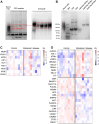
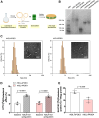
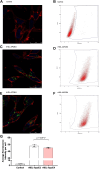
In the central nervous system, apolipoprotein (APO)E-containing lipoprotein particles mediate the transport of glial-derived cholesterol to neurons, which is essential for neuronal membrane remodeling and maintenance of the myelin sheath. We aimed to examine cholesterol transport via lipoprotein particles in cerebrospinal fluid (CSF) of Alzheimer's disease (AD) patients compared to control individuals. Additionally, we explored the ability of reconstituted HDL containing different APOE isoforms to regulate cholesterol transport. We evaluated the capacity of CSF lipoprotein particles to facilitate radiolabeled unesterified cholesterol efflux from A172 human glioblastoma astrocytes and to deliver cholesterol to SH-SY5Y human neuronal cells. The CSF lipoprotein proteome was analyzed by LC-MS/MS. Reconstituted HDL nanoparticles were prepared by combining phospholipids and cholesterol with human APOE3 or APOE4, followed by radiolabeling with unesterified cholesterol. Our results showed that cholesterol efflux from astrocytes to CSF were similar between AD patients and controls, both under baseline conditions and after activation of ABCA1 and ABCG1. However, CSF lipoprotein-mediated neuronal cholesterol uptake was significantly reduced in the AD group. LC-MS/MS analysis identified 239 proteins associated with CSF lipoproteins in both groups, with no major alterations in proteins linked to cholesterol metabolism. However, 27 proteins involved in noncholesterol-related processes were differentially expressed. Notably, synthetic reconstituted HDL particles containing APOE4 exhibited reduced capacity to deliver cholesterol to neurons compared to those with APOE3. These findings indicate that CSF lipoproteins from patients with AD demonstrate impaired cholesterol delivery to neurons. Our study highlights APOE4 as a critical contributor to abnormal neuronal cholesterol uptake in AD pathophysiology.
Keywords: APOE; Alzheimer's disease; CSF lipoprotein; cholesterol.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest J. F. reported receiving personal fees for service on the advisory boards, adjudication committees or speaker honoraria from AC Immune, Adamed, Alzheon, Biogen, Eisai, Esteve, Fujirebio, Ionis, Laboratorios Carnot, Life Molecular Imaging, Lilly, Lundbeck, Perha, Roche, Zambón and outside the submitted work. J. F. reports holding a patent for markers of synaptopathy in neurodegenerative disease (licensed to ADx, EPI8382175.0). The other authors declare that they have no conflicts of interest with the contents of this article.
Figures

Update of
-
Impaired Cerebrospinal Fluid Lipoprotein-Mediated Cholesterol Delivery to Neurons in Alzheimer's Disease.Res Sq [Preprint]. 2024 Dec 23:rs.3.rs-5682870. doi: 10.21203/rs.3.rs-5682870/v1. Res Sq. 2024. Update in: J Lipid Res. 2025 Aug;66(8):100865. doi: 10.1016/j.jlr.2025.100865. PMID: 39764088 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

