Safety of mycophenolate mofetil in systemic lupus erythematosus maintenance therapy: insights from the LUNA registry in a nationwide prospective cohort study
- PMID: 40701623
- PMCID: PMC12306299
- DOI: 10.1136/rmdopen-2025-005558
Safety of mycophenolate mofetil in systemic lupus erythematosus maintenance therapy: insights from the LUNA registry in a nationwide prospective cohort study
Abstract
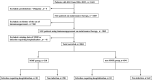
Objective: We aimed to compare the incidence of severe infections between mycophenolate mofetil (MMF) and other immunosuppressants in patients with systemic lupus erythematosus (SLE) on maintenance therapy using data from the Lupus Registry of Nationwide Institutions in Japan.
Methods: This study employed a prospective cohort design, including patients with SLE undergoing maintenance therapy. Exposure was defined as MMF treatment, and the control group included individuals who received other immunosuppressants (non-MMF) treatments. Severe infections requiring hospitalisation were the primary outcomes, whereas secondary outcomes included all-cause hospitalisation, changes in prednisolone dosage, disease activity and organ damage. Statistical analyses employed marginal structural models with stabilised inverse probability of treatment weighting to adjust for confounders.
Results: The analysis included 1004 patients; the incidence of severe infections was 6.5% in the MMF group and 7.5% in the non-MMF group, with no significant difference (OR 0.69, 95% CI 0.34 to 1.39). Similarly, all-cause hospitalisation rates were comparable between the groups (OR 0.72, 95% CI 0.47 to 1.09). Prednisolone dosage was significantly reduced in the MMF group (-0.66 mg/day, 95% CI -1.09 to -0.23). The organ damage score was modestly reduced in the MMF group (-0.19 points, 95% CI -0.37 to -0.01), whereas the change of disease activity score was comparable between the groups (-0.10 points, 95% CI -0.74 to 0.55).
Conclusions: MMF does not significantly increase the risk of severe infections compared with other immunosuppressants in SLE maintenance therapy. MMF may contribute to reducing the dose of prednisolone.
Keywords: Autoimmune Diseases; Lupus Erythematosus, Systemic; Treatment.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Immunosuppressive treatment for proliferative lupus nephritis.Cochrane Database Syst Rev. 2018 Jun 29;6(6):CD002922. doi: 10.1002/14651858.CD002922.pub4. Cochrane Database Syst Rev. 2018. PMID: 29957821 Free PMC article.
-
Immunosuppressants for the prophylaxis of corneal graft rejection after penetrating keratoplasty.Cochrane Database Syst Rev. 2015 Aug 27;2015(8):CD007603. doi: 10.1002/14651858.CD007603.pub2. Cochrane Database Syst Rev. 2015. PMID: 26313245 Free PMC article.
-
Clinical and cost-effectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study.Health Technol Assess. 2005 May;9(21):1-179, iii-iv. doi: 10.3310/hta9210. Health Technol Assess. 2005. PMID: 15899149
-
Treatment for lupus nephritis.Cochrane Database Syst Rev. 2012 Dec 12;12:CD002922. doi: 10.1002/14651858.CD002922.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2018 Jun 29;6:CD002922. doi: 10.1002/14651858.CD002922.pub4. PMID: 23235592 Updated.
-
Mycophenolic acid versus azathioprine as primary immunosuppression for kidney transplant recipients.Cochrane Database Syst Rev. 2015 Dec 3;2015(12):CD007746. doi: 10.1002/14651858.CD007746.pub2. Cochrane Database Syst Rev. 2015. PMID: 26633102 Free PMC article.
References
-
- New ACR guideline summary provides guidance to screen, treat, and manage lupus nephritis. 2024. https://rheumatology.org/press-releases/new-acr-guideline-summary-provid... Available.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical