Mitochondrial dysfunction drives basal cell hyperplasia in eosinophilic oesophagitis
- PMID: 40701793
- PMCID: PMC12438975
- DOI: 10.1136/gutjnl-2024-334561
Mitochondrial dysfunction drives basal cell hyperplasia in eosinophilic oesophagitis
Abstract
Background: Eosinophilic oesophagitis (EoE) is a food allergen-induced inflammatory disorder characterised by interleukin (IL)-13-mediated oesophageal inflammation and epithelial basal cell hyperplasia (BCH). The role of mitochondria in EoE pathogenesis remains elusive.
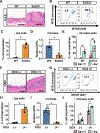
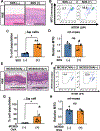
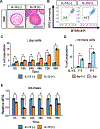
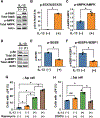
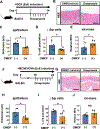
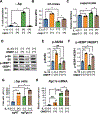
Design: Prompted by single cell transcriptomics data, we interrogated the role of mitochondria in EoE pathobiology using patient biopsies, EoE-mouse models and oesophageal epithelial cells grown in monolayer and three-dimensional (3D) organoid cultures treated with EoE-relevant cytokines. 3D organoids and EoE-bearing mice were treated with omeprazole-a proton-pump inhibitor used as first-line EoE therapy. We performed CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) interference in mouse organoids to identify the key mitochondrial regulatory genes whose depletion may lead to BCH. We analysed mitochondrial membrane potential, mass and superoxide production by flow cytometry, cellular oxygen consumption by respirometry, mitochondrial structures and perturbation of cellular energy homeostasis by immunoblotting. RESULTS : Mitochondrial dysfunction appeared to be a hallmark of EoE-related BCH where mitochondrial structural damage was associated with impaired oxidative respiratory capacity, elevation of mitochondrial superoxide and decreased adenosine triphosphate (ATP) production, as corroborated by activation of the adenosine monophosphate (AMP) -activated protein kinase and suppression of mammalian target-of-rapamycin signalling. Depletion of PGC1A, the master regulator of mitochondria biogenesis, recapitulated EoE-related BCH, suggesting that mitochondrial dysfunction drives BCH. Further, omeprazole alleviated mitochondrial damage and dysfunction in EoE-related BCH modelled in mice and patient-derived organoids. CONCLUSION: Mitochondrial dysfunction is tightly linked to perturbation of redox homeostasis in EoE-related BCH, which is promoted by IL-13 and reversible with omeprazole treatment.
Keywords: GASTROINTESTINAL IMMUNE RESPONSE; INFLAMMATORY DISEASES; OESOPHAGITIS.
© Author(s) (or their employer(s)) 2025. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures






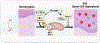
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
