Physical Exercise Improves Symptomatic Dermographism
- PMID: 40701931
- PMCID: PMC12286705
- DOI: 10.1002/clt2.70083
Physical Exercise Improves Symptomatic Dermographism
Abstract
Background: Short-term exercise may reduce disease activity in symptomatic dermographism (SD), but its prevalence and short- and long-term effects remain unclear and understudied. This study aims to assess the impact of both short-term and regular long-term exercise programs on disease activity in patients with SD.
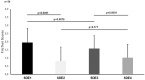
Methods: We performed a short-term exercise test to assess the disease activity and the critical friction threshold (CFT) using the FricTest before (SDE1) and 10 min after (SDE2) this test on 34 SD patients. Afterward, we asked the patients to carry on a 1-month regular long-term exercise program according to the World Health Organization's physical activity recommendations. At the end of this 1-month period, we performed the short-term exercise test using the FricTest before (SDE3) and 10 min after (SDE4) the exercise test.
Results: Before a 1-month regular exercise program, 32 of 34 patients (94.1%) showed a reduction in the critical friction threshold after the short-term exercise test (SDE1; 1.95 ± 0.88 vs. SDE2; 0.81 ± 0.86). After a 1-month regular exercise program, 29 of 34 patients (85%) showed a reduction in SD symptoms with short-term exercise test and the FricTest scores were significantly decreased (SDE3; 1.57 ± 0.80 vs. SDE4; 1.01 ± 0.83). After the 1-month regular exercise program, a statistically significant increase was seen in the patients' UCT scores and quality of life.
Conclusions: Our findings show that short-term exercise improves SD symptoms, while long-term regular exercise programs reduce disease symptoms and improve UCT scores and quality of life.
Keywords: chronic urticaria; exercise; physical activity; symptomatic dermographism.
© 2025 The Author(s). Clinical and Translational Allergy published by John Wiley & Sons Ltd on behalf of European Academy of Allergy and Clinical Immunology.
Conflict of interest statement
R.E. was a speaker and/or advisor for Novartis, Abbvie, Jannsen, and Pfizer. M.B.Y. and Ş.K.E. do not have any conflict of interest to report. M.T. is or recently was a speaker and/or advisor for AstraZeneca, Chiesi, GSK, Novartis, ROXALL, Vem İlaç. E.K. is/was recently a speaker/consultant for Menarini and Novartis and received research funding from Almirall. M.M. is or recently was a speaker and/or advisor for and/or has received research funding from Celltrion, Jasper Therapeutics, Blueprint, Takeda, Biocryst, Annexon, and Roche outside of the submitted manuscript.
Figures

References
-
- Ornek O. S., Kuteyla C. P., Degirmentepe E. N., Cure K., Singer R., and Kocaturk E., “A Comparative Analysis of Chronic Inducible Urticaria in 423 Patients: Clinical and Laboratory Features and Comorbid Conditions,” Journal of the European Academy of Dermatology and Venereology 38, no. 3 (2024): 513–520, 10.1111/jdv.19637. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

