Crystallizing the Uncrystallizable: Insights from Extensive Screening of PROTACs
- PMID: 40701949
- PMCID: PMC12333357
- DOI: 10.1021/jacs.5c07977
Crystallizing the Uncrystallizable: Insights from Extensive Screening of PROTACs
Abstract
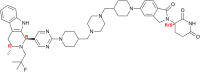
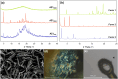
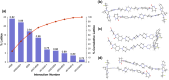
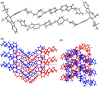
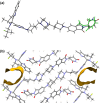
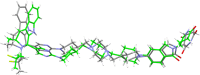
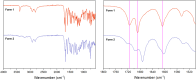
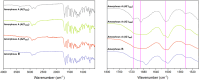
PROTACs are new drug molecules in the beyond Rule of Five (bRo5) chemical space with extremely poor aqueous solubility and intrinsically poor crystallizability due to their structure, which comprises two distinct ligands covalently linked by a flexible linker. This makes PROTACs particularly challenging to understand from a solid-state preformulation perspective. While several X-ray structures have been reported of PROTACs in ternary complexes, to date no structures have been published of single component densely packed PROTACs, from which an understanding of PROTACs' intermolecular interactions, and therefore physical properties, can be developed. An extensive crystallization protocol was applied to grow single crystals of a cereblon-recruiting PROTAC "AZ1" resulting in structures of an anhydrous form and a nonstoichiometric p-xylene solvate using 3D electron diffraction and synchrotron X-ray crystallography, respectively. The lattice energies are dominated by dispersive interactions between AZ1 molecules despite the presence of multiple hydrogen-bond donors and acceptors and planar aromatic groups, and both structures are built on similar intermolecular interactions. Thermal and spectral characterization revealed another solvate form containing dichloromethane. Amorphous solids produced by mechanochemical grinding of anhydrous AZ1 crystals also differed in dissolution characteristics from an amorphous solid produced by desolvating the dichloromethane solvate crystals, indicating that AZ1 may demonstrate pseudo-polyamorphism. This study paves the way for solid form screening and understanding in pharmaceutical systems that are far bRo5.
Figures





References
-
- Brittain, H. G. Polymorphism in Pharmaceutical Solids; Dekker: New York, 1999; Vol. 95.
-
- Saifee M., Inamdar N., Dhamecha D. L., Rathi A. A.. Drug polymorphism: a review. Int. J. Health Res. 2010;2:291–306. doi: 10.4314/ijhr.v2i4.55423. - DOI
-
- Suresh K., Mehta T., Thakrar V., Sharma R. G.. Innovative Strategies in Generic Drug Development: The Role of Polymorph, Amorphous, Pseudopolymorph, and Cocrystal Solid Forms. Cryst. Growth Des. 2025;25:1282–1292. doi: 10.1021/acs.cgd.4c01558. - DOI
LinkOut - more resources
Full Text Sources

