H3K14 lactylation exacerbates neuronal ferroptosis by inhibiting calcium efflux following intracerebral hemorrhagic stroke
- PMID: 40701963
- PMCID: PMC12287320
- DOI: 10.1038/s41419-025-07874-9
H3K14 lactylation exacerbates neuronal ferroptosis by inhibiting calcium efflux following intracerebral hemorrhagic stroke
Abstract
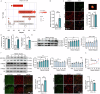
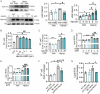
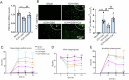
Inhibiting neuronal ferroptosis is essential for mitigating neural damage and enhancing recovery in central nervous system (CNS) disorders, including intracerebral hemorrhagic stroke (ICH). Lactate accumulation correlates with ICH severity, yet the role of lactate-derived histone lactylation, a novel epigenetic modification, in ferroptosis and its mechanisms is not fully understood. In this study, we aimed to investigate the role of histone lactylation on neuronal ferroptosis in ICH models, both in vitro and in vivo. We discovered elevated lactate and histone lactylation post-ICH in mice, with a significant increase in H3K14la during the early stages of ferroptosis in hemin-challenged primary cortical neurons. Pharmacological or genetic inhibition of H3K14la by targeting lactate dehydrogenase (LDH) enzyme activity effectively suppressed neuronal ferroptosis. We further identified p300/CBP and class I histone deacetylases (HDACs) as the key modifiers of H3K14la in this process. Through chromatin immunoprecipitation-sequencing and RNA-sequencing (RNA-seq) in hemin-treated neurons, we pinpointed the Ca2+-ATPase PMCA2 encoding gene as a direct downstream target of H3K14la. H3K14la/PMCA2 promoted ferroptosis by elevating intracellular calcium levels. In line with our in vitro findings, inhibiting H3K14la/PMCA2 reduced neuronal degeneration and improved functional outcomes in an ICH mouse model induced by intracranial injection of collagenase into the striatum. Taken together, our findings elucidate the role of histone lactylation and PMCA2 in neuronal ferroptosis and implicate that targeting histone lactylation could be a promising therapeutic strategy for ICH and related CNS diseases.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: All animal experiments were approved by the Animal Care and Use Committee of Capital Medical University (AEEI-2020-164). All methods were performed in accordance with the relevant guidelines and regulations.
Figures




Similar articles
-
miR-210 Regulates Autophagy Through the AMPK/mTOR Signaling Pathway, Reduces Neuronal Cell Death and Inflammatory Responses, and Enhances Functional Recovery Following Cerebral Hemorrhage in Mice.Neurochem Res. 2025 Jun 5;50(3):180. doi: 10.1007/s11064-025-04434-7. Neurochem Res. 2025. PMID: 40471451 Free PMC article.
-
BRD4 silencing attenuates hemin-induced neuronal ferroptosis and inflammation via the H3K27ac-ATF3 axis in an in vitro model of cerebral hemorrhage.Eur J Med Res. 2025 Jul 2;30(1):556. doi: 10.1186/s40001-025-02759-1. Eur J Med Res. 2025. PMID: 40605044 Free PMC article.
-
The histone lactylation of AIM2 influences the suppression of ferroptosis by ACSL4 through STAT5B and promotes the progression of lung cancer.FASEB J. 2025 Jan 15;39(1):e70308. doi: 10.1096/fj.202402139R. FASEB J. 2025. PMID: 39792364
-
Research progress on the interaction between glucose metabolic reprogramming and lactylation in tumors.Front Immunol. 2025 Jul 14;16:1595162. doi: 10.3389/fimmu.2025.1595162. eCollection 2025. Front Immunol. 2025. PMID: 40755753 Free PMC article. Review.
-
Antithrombotic treatment after stroke due to intracerebral haemorrhage.Cochrane Database Syst Rev. 2023 Jan 26;1(1):CD012144. doi: 10.1002/14651858.CD012144.pub3. Cochrane Database Syst Rev. 2023. PMID: 36700520 Free PMC article.
References
-
- Lan T, Sun TT, Wei C, Cheng T, Yang F, Zhang J-N, et al. Epigenetic regulation of ferroptosis in central nervous system diseases. Mol Neurobiol. 2023;60:3584–99. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

