Nipah virus vaccines evaluated in pigs as a 'One Health' approach to protect public health
- PMID: 40702012
- PMCID: PMC12287429
- DOI: 10.1038/s41541-025-01212-y
Nipah virus vaccines evaluated in pigs as a 'One Health' approach to protect public health
Abstract
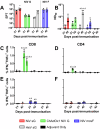
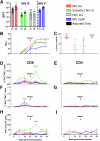
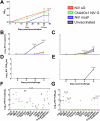
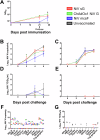
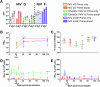
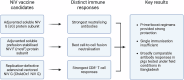
Nipah virus (NiV) causes a severe neurological disease in humans. The first NiV outbreak, in Malaysia, involved pig-to-human transmission, that resulted in significant economic losses to the local pig industry. Despite the risk NiV poses to pig-dense regions, no licensed vaccines exist. This study therefore assessed three NiV vaccine candidates in pigs: (1) adjuvanted soluble NiV (s)G protein, (2) adjuvanted pre-fusion stabilised NiV (mcs)F protein, and (3) adenoviral vectored NiV G (ChAdOx1 NiV G). NiV sG induced the strongest neutralising antibody response, NiV mcsF induced antibodies best able to neutralise cell-cell fusion, whereas ChAdOx1 NiV G elicited CD8+ T-cell responses. Despite differences in immunogenicity, prime-boost immunisation with all candidates conferred a high degree of protection against NiV infection. Follow-up studies demonstrated longevity of immune responses and broadly comparable immune responses in Bangladeshi pigs under field conditions. These studies provide a platform for developing a NiV vaccine for pigs.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: S.C.G. is a cofounder of and shareholder in Vaccitech and named as an inventor on a patent covering use of ChAdOx1-vectored vaccines (PCT/GB2012/000467). T.L. is named as an inventor on a patent covering use of ChAdOx1-vectored vaccines (PCT/GB2012/000467) and was a consultant to Vaccitech. M.M. and R.R. are/were employees of Zoetis, respectively. All other authors declare no competing interests.
Figures

References
-
- Escaffre, O., Borisevich, V. & Rockx, B. Pathogenesis of Hendra and Nipah virus infection in humans. J. Infect. Dev. Ctries7, 308–311 (2013). - PubMed
-
- Tan, K.-S., Tan, C. T. & Goh, K. J. Epidemiological aspects of Nipah virus infection. Neurol. J. Southeast Asia4, 77–81 (1999).
-
- Luby, S. P. & Gurley, E. S. Epidemiology of henipavirus disease in humans. Curr. Top. Microbiol. Immunol.359, 25–40 (2012). - PubMed
-
- Clayton, B. A. Nipah virus: transmission of a zoonotic paramyxovirus. Curr. Opin. Virol.22, 97–104 (2017). - PubMed
Grants and funding
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- 971555/Innovate UK,United Kingdom
- BBS/E/I/00007031/Biotechnology and Biological Sciences Research Council,United Kingdom
- BBS/E/I/00007031/Biotechnology and Biological Sciences Research Council,United Kingdom
- BBS/E/I/00007031/Biotechnology and Biological Sciences Research Council,United Kingdom
- BBS/E/I/00007031/Biotechnology and Biological Sciences Research Council,United Kingdom
- BBS/E/I/00007031/Biotechnology and Biological Sciences Research Council,United Kingdom
- BBS/E/I/00007031/Biotechnology and Biological Sciences Research Council,United Kingdom
- BBS/E/I/00007031/Biotechnology and Biological Sciences Research Council,United Kingdom
- BBS/E/I/00007031/Biotechnology and Biological Sciences Research Council,United Kingdom
- BBS/E/I/00007039/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00007039/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00007039/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00007039/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00007039/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00007039/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00007039/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00007039/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Research Materials

