Study of antibacterial activity of copper zinc nanocomposites and disruption of bacterial cytoplasmic membrane
- PMID: 40702152
- PMCID: PMC12287259
- DOI: 10.1038/s41598-025-93691-1
Study of antibacterial activity of copper zinc nanocomposites and disruption of bacterial cytoplasmic membrane
Abstract
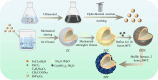
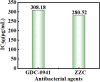
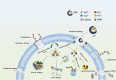
In recent years, the widespread use of antibiotics has led to the emergence of numerous drug-resistant bacteria, posing a severe threat to both human health and the economy. As a result, it is imperative to develop efficient antibacterial agents that do not induce drug resistance. This study employed layer-by-layer assembly technology to prepare ZnFe2O4@ZnS/Cu2S (ZZC) nanocomposites Gram-negative Escherichia coli (E. coli), Gram-positive Staphylococcus aureus (S. aureus) and drug-resistant Salmonella (T-Salmonella) were utilized as test bacteria to investigate the antibacterial effectiveness and mechanism of ZZC. The findings demonstrated that, the MIC of the ZZC against E. coli, S. aureus and T-Salmonella were 50, 60 and 80 μg/mL, respectively; At a material concentration of 200 μg/mL and a reaction time of 80 min, ZZC demonstrated a bacteriostatic rate of 99.99% against the three tested bacteria. The nano-composite can disrupt cell walls and plasma membranes and effectively and resulting in bacterial rupture and demise. Furthermore, the nano-composite displayed strong biocompatibility and was also able to heal mixed bacterial-induced wound infections and essentially eliminated the bacterial burden after 9 days, and also exhibited excellent antimicrobial activity in vivo. The results also indicate significant potential for its application in medical materials and other areas of research.
Keywords: Antibacterial mechanism; Bacteriostatic materials; Healing of wound; Nano-composites; ZnFe2O4.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: All animal experiments were conducted in line with the regulations of Hanzhong Central Hospital and the ARRIVE Guidelines for Reporting Animal Research. In these experiments, tribromoethanol was used to administer an overdose of anesthesia to euthanize the animals. The experimental protocol has been approved by the Animal Ethics Committees of Hanzhong Central Hospital and Xijing Hospital of Air Force Medical University (Approval No. 108-20240324).
Figures









References
-
- Friedman, N. D., Temkin, E. & Carmeli, Y. The negative impact of antibiotic resistance. Clin. Microbiol. Infect.22, 416–422. 10.1016/j.cmi.2015.12.002 (2016). - PubMed
-
- Gong, X. et al. An overview of green synthesized silver nanoparticles towards bioactive antibacterial, antimicrobial and antifungal applications. Adv. Colloid Interface Sci.323, 103053. 10.1016/j.cis.2023.103053 (2024). - PubMed
-
- Guo, S. et al. Antibacterial effect of the metal nanocomposite on Escherichia coli. J. Hazardous Mater.476, 135149. 10.1016/j.jhazmat.2024.135149 (2024). - PubMed
-
- Xu, P. et al. Microfluidic controllable synthesis of monodispersed sulfur nanoparticles with enhanced antibacterial activities[J]. Chem. Eng. J.398, 125293. 10.1016/j.cej.2020.125293 (2020).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

