Topical ophthalmic administration of the antiangiogenic peptide VIAN-c4551 protects against experimental diabetic macular edema
- PMID: 40702159
- PMCID: PMC12287436
- DOI: 10.1038/s41598-025-12331-w
Topical ophthalmic administration of the antiangiogenic peptide VIAN-c4551 protects against experimental diabetic macular edema
Abstract
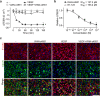
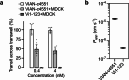
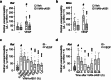
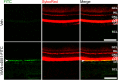
Increased angiogenesis and vascular permeability are hallmarks of microvascular retinal diseases such as diabetic retinopathy and diabetic macular edema (DME). Periodic intravitreal injections of inhibitors of the vascular endothelial growth factor (VEGF) are first-line therapy, but their invasiveness and associated risks often lead to poor compliance and outcomes. Here, we investigate VIAN-c4551, a highly potent antiangiogenic cyclic heptapeptide, as a non-invasive topical ophthalmic alternative to the current standard of care for DME. VIAN-c4551 demonstrated high potency (IC50 = 137 pM) to inhibit the permeability of human umbilical vein endothelial cell monolayers induced by VEGF. VIAN-c4551 eye drops potently (0.005% minimum effective dose) prevented, for up to 24 h, the retinal vascular leakage induced by VEGF injected intravitreally and reversed the increased retinal vascular permeability due to diabetes in rats and mice. VIAN-c4551 exhibited high penetrability across MDCK epithelium and, after a single eye drop in rabbits, reached the vitreous and the retina-choroid at concentrations several orders of magnitude above its IC50 (Cmax ~239 nM and ~ 6.7 µM, respectively, after 6 h) that lasted at least 24 h. In conclusion, VIAN-c4551 is a non-invasive, once-a-day potential intervention for preventing and reversing retinal vascular leakage in DME and other vascular retinopathies and preserving sight.
Keywords: Anti-angiogenesis; Diabetic macular edema; Diabetic retinopathy; Efficacy; Eye drops; Pharmacokinetics; Therapeutic peptide; Vascular permeability; Vasoinhibin analog.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: JPR, MZ, TB, JT, GME, and CC are inventors of a submitted patent application (WO/2021/098996), which is owned by the Universidad Nacional Autónoma de México (UNAM) and JT. JPR is the CEO and founder of VIAN Therapeutics Inc. MZ and CC are consultants for VIAN Therapeutics. Inc.
Figures

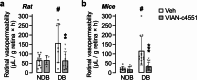

References
-
- Nguyen, Q. D. et al. Vascular endothelial growth factor is a critical stimulus for diabetic macular edema. Am. J. Ophthalmol.142, 961–969 (2006). - PubMed
MeSH terms
Substances
Grants and funding
- SECTEI/061/2023/Secretaría de Educación, Ciencia, Tecnología e Innovación de la Ciudad de México
- SECTEI/061/2023/Secretaría de Educación, Ciencia, Tecnología e Innovación de la Ciudad de México
- SECTEI/061/2023/Secretaría de Educación, Ciencia, Tecnología e Innovación de la Ciudad de México
- SECTEI/061/2023/Secretaría de Educación, Ciencia, Tecnología e Innovación de la Ciudad de México
- SECTEI/061/2023/Secretaría de Educación, Ciencia, Tecnología e Innovación de la Ciudad de México
- SECTEI/061/2023/Secretaría de Educación, Ciencia, Tecnología e Innovación de la Ciudad de México
- SECTEI/061/2023/Secretaría de Educación, Ciencia, Tecnología e Innovación de la Ciudad de México
- SECTEI/061/2023/Secretaría de Educación, Ciencia, Tecnología e Innovación de la Ciudad de México
- SECTEI/061/2023/Secretaría de Educación, Ciencia, Tecnología e Innovación de la Ciudad de México
- SECTEI/061/2023/Secretaría de Educación, Ciencia, Tecnología e Innovación de la Ciudad de México
- SECTEI/061/2023/Secretaría de Educación, Ciencia, Tecnología e Innovación de la Ciudad de México
- SECTEI/061/2023/Secretaría de Educación, Ciencia, Tecnología e Innovación de la Ciudad de México
LinkOut - more resources
Full Text Sources
Medical

