Mechanical underwater adhesive devices for soft substrates
- PMID: 40702173
- PMCID: PMC12310550
- DOI: 10.1038/s41586-025-09304-4
Mechanical underwater adhesive devices for soft substrates
Abstract
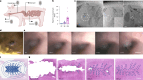
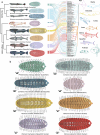
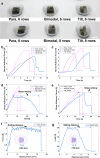
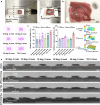
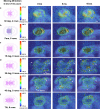
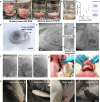
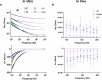
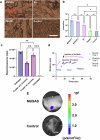
Achieving long-term underwater adhesion to dynamic, regenerating soft substrates that undergo extreme fluctuations in pH and moisture remains a major unresolved challenge, with far-reaching implications for healthcare, manufacturing, robotics and marine applications1-16. Here, inspired by remoras-fish equipped with specialized adhesive discs-we developed the Mechanical Underwater Soft Adhesion System (MUSAS). Through detailed anatomical, behavioural, physical and biomimetic investigations of remora adhesion on soft substrates, we uncovered the key physical principles and evolutionary adaptations underlying their robust attachment. These insights guided the design of MUSAS, which shows extraordinary versatility, adhering securely to a wide range of soft substrates with varying roughness, stiffness and structural integrity. MUSAS achieves an adhesion-force-to-weight ratio of up to 1,391-fold and maintains performance under extreme pH and moisture conditions. We demonstrate its utility across highly translational models, including in vitro, ex vivo and in vivo settings, enabling applications such as ultraminiaturized aquatic kinetic temperature sensors, non-invasive gastroesophageal reflux monitoring, long-acting antiretroviral drug delivery and messenger RNA administration via the gastrointestinal tract.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Z.K., G.T., A.R.K., M.Z. and Y.C. are co-inventors on a patent application describing the system reported: US Provisional Application No. 63/702,561. Complete details of all relationships for profit and not for profit for G.T. can found at https://www.dropbox.com/sh/szi7vnr4a2ajb56/AABs5N5i0q9AfT1IqIJAE-T5a?dl=0 . The other authors declare no competing interests.
Figures







References
-
- Rich, S. I., Wood, R. J. & Majidi, C. Untethered soft robotics. Nat. Electron.1, 102–112 (2018).
-
- Martinez, R. V. et al. Robotic tentacles with three-dimensional mobility based on flexible elastomers. Adv. Mater.25, 205–212 (2013). - PubMed
-
- Fan, H. & Gong, J. P. Bioinspired underwater adhesives. Adv. Mater.33, 2102983 (2021). - PubMed
-
- Soltannia, B. & Sameoto, D. Strong, reversible underwater adhesion via gecko-inspired hydrophobic fibers. ACS Appl. Mater. Interfaces6, 21995–22003 (2014). - PubMed
-
- Wang, Y. et al. A biorobotic adhesive disc for underwater hitchhiking inspired by the remora suckerfish. Sci. Robot.2, eaan8072 (2017). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

