Nanobody therapy rescues behavioural deficits of NMDA receptor hypofunction
- PMID: 40702184
- PMCID: PMC12408334
- DOI: 10.1038/s41586-025-09265-8
Nanobody therapy rescues behavioural deficits of NMDA receptor hypofunction
Abstract
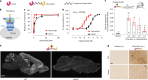
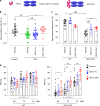
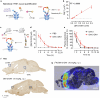
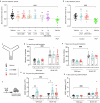
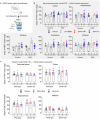
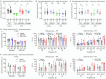
There is an urgent need for efficient and innovative therapies to treat brain disorders such as psychiatric and neurodegenerative diseases. Immunotherapies have proved to be efficient in many medical areas, but have not been considered to treat brain diseases due to the poor brain penetration of immunoglobulins1,2. Here we developed a bivalent biparatopic antibody, made of two camelid heavy-chain antibodies (called nanobodies)3, one binding to, and the other potentiating the activity of, homodimeric metabotropic glutamate receptor 2. We show that this bivalent nanobody, given peripherally, reaches the brain and corrects cognitive deficits in two preclinical mouse models with endophenotypes resulting from NMDA receptor hypofunction. Notably, these in vivo effects last for at least 7 days after a single intraperitoneal injection and are maintained after subchronic treatment. Our results establish a proof of concept that nanobodies can target brain receptors, and pave the way for nanobody-based therapeutic strategies for the treatment of brain disorders.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors have related patents granted under patent numbers WO2016001417A1 (J.-P.P., P.C. and P.R.), WO2024003389A1 (J.-P.P., P.R., P.C., J.K., M.O., A.R. and C.B.) and WO2024003390A1 (J.-P.P., P.R., L.P., J.K., M.O. and M.T.). The other authors declare no competing interests.
Figures









References
-
- Terstappen, G. C., Meyer, A. H., Bell, R. D. & Zhang, W. Strategies for delivering therapeutics across the blood-brain barrier. Nat. Rev. Drug. Discov.20, 362–383 (2021). - PubMed
-
- Sevigny, J. et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature537, 50–56 (2016). - PubMed
-
- Heneka, M. T., Morgan, D. & Jessen, F. Passive anti-amyloid β immunotherapy in Alzheimer’s disease-opportunities and challenges. Lancet404, 2198–2208 (2024). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

