Global hotspots of mycorrhizal fungal richness are poorly protected
- PMID: 40702191
- PMCID: PMC12422971
- DOI: 10.1038/s41586-025-09277-4
Global hotspots of mycorrhizal fungal richness are poorly protected
Abstract
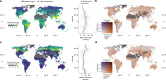
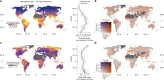
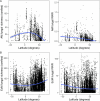
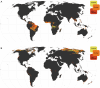
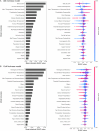
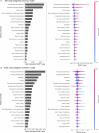
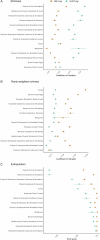
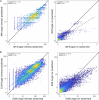
Mycorrhizal fungi are ecosystem engineers that sustain plant life and help regulate Earth's biogeochemical cycles1-3. However, in contrast to plants and animals, the global distribution of mycorrhizal fungal biodiversity is largely unknown, which limits our ability to monitor and protect key underground ecosystems4,5. Here we trained machine-learning algorithms on a global dataset of 25,000 geolocated soil samples comprising >2.8 billion fungal DNA sequences. We predicted arbuscular mycorrhizal and ectomycorrhizal fungal richness and rarity across terrestrial ecosystems. On the basis of these predictions, we generated high-resolution, global-scale maps and identified key reservoirs of highly diverse and endemic mycorrhizal communities. Intersecting protected areas with mycorrhizal hotspots indicated that less than 10% of predicted mycorrhizal richness hotspots currently exist in protected areas. Our results describe a largely hidden component of Earth's underground ecosystems and can help identify conservation priorities, set monitoring benchmarks and create specific restoration plans and land-management strategies.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: E.T.K. and C.A. are the founders of the Society for the Protection of Underground Networks (SPUN), a non-governmental organization (NGO) that conducts research on mycorrhizal fungi for conservation and restoration. T.W.C. is the founder of Restor, an NGO that facilitates the global restoration movement. C.A. is the founder of Funga, an organization that facilitates the restoration of belowground fungal biodiversity. G.F. is the founding director of Fungi Foundation, an NGO that explores and educates about fungal diversity for applications and conservation. M.E.V.N., J.D.S., O.P., A.C., L.G.v.G., B.F.M., C.Q., T.L., V.M., O.D., C.M., M.S., J.W., K.G.P., C.K.C., T.V., P.K., P.B., L.T., S.A.W. and J.v.d.H. declare no competing interests.
Figures






References
-
- Högberg, M. N. & Högberg, P. Extramatrical ectomycorrhizal mycelium contributes one-third of microbial biomass and produces, together with associated roots, half the dissolved organic carbon in a forest soil. New Phytol.154, 791–795 (2002). - PubMed
-
- van Der Heijden, M. G., Martin, F. M., Selosse, M. A. & Sanders, I. R. Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol.205, 1406–1423 (2015). - PubMed
-
- Hawkins, H. J. et al. Mycorrhizal mycelium as a global carbon pool. Curr. Biol.33, R560–R573 (2023). - PubMed
-
- Guerra, C. A. et al. Tracking, targeting, and conserving soil biodiversity. Science371, 239–241 (2021). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

