Upregulation of adenosine A2A receptor in astrocytes is sufficient to trigger hippocampal multicellular dysfunctions and memory deficits
- PMID: 40702259
- PMCID: PMC12532706
- DOI: 10.1038/s41380-025-03115-9
Upregulation of adenosine A2A receptor in astrocytes is sufficient to trigger hippocampal multicellular dysfunctions and memory deficits
Abstract
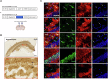
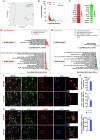
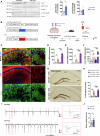
Adenosine is an ubiquitous neuromodulator that ensures cerebral homeostasis. It exerts numerous functions through the activation of G-protein-coupled adenosine receptors (ARs), in particular A1 (A1R) and A2A (A2AR) receptors. Interestingly, A2AR levels are upregulated in cortical and hippocampal regions in several pathological conditions such as Alzheimer's disease, tauopathies or epilepsia. Such abnormal upregulations have been particularly reported in astrocytes, glial cells that play a key role in regulating synaptic plasticity. However, the overall impact and the underlying mechanisms associated with increased A2AR in astrocytes remain poorly understood. In the present study, we induced the upregulation of A2AR in hippocampal astrocytes using dedicated AAVs and comprehensively evaluated the functional consequences in 4 months-old C57Bl6/J mice. Our results show that A2AR upregulation primarily promotes alterations of astrocyte reactivity, morphology and transcriptome, with a link to aging-like phenotype as well as secondary impairments of neuronal excitability and microglial phenotype. These changes driven by a restricted A2AR upregulation in hippocampal astrocytes were sufficient to induce impairments of short-term spatial memory and spatial learning. This study highlights the impact of astrocytic A2AR upregulation, as seen in various neurological conditions, on the development of a detrimental multicellular response associated with memory alterations and provides an additional proof-of-concept for the value of targeting this receptor in different neurodegenerative conditions.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: DB is a (non-appointed) member of the scientific advisory board of Marvel Biosciences Corp developing an A2AR antagonist. But there is no conflict of interest regarding the present work. Other authors have no competing financial interests in relation to the work described.
Figures



References
-
- Cunha RA. How does adenosine control neuronal dysfunction and neurodegeneration? J Neurochem. 2016;139:1019–55. - PubMed
-
- Rebola N, Lujan R, Cunha RA, Mulle C. Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron. 2008;57:121–34. - PubMed
-
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul J-Y, et al. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–6. - PubMed
-
- Matos M, Augusto E, Santos-Rodrigues AD, Schwarzschild MA, Chen J-F, Cunha RA, et al. Adenosine A2A receptors modulate glutamate uptake in cultured astrocytes and gliosomes. Glia. 2012;60:702–16. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

