Uridine phosphorylase-1 supports metastasis by altering immune and extracellular matrix landscapes
- PMID: 40702342
- PMCID: PMC12420820
- DOI: 10.1038/s44319-025-00520-7
Uridine phosphorylase-1 supports metastasis by altering immune and extracellular matrix landscapes
Abstract
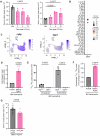
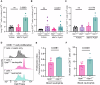
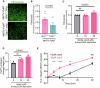
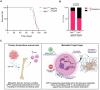
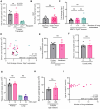
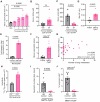
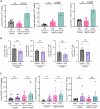
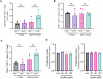
Understanding mechanisms that facilitate early events in metastatic seeding is key to developing therapeutic approaches to reduce metastasis. Here we identify uracil as a metastasis-associated metabolite in genetically engineered mouse models of cancer and in patients with metastatic breast cancer. Uracil is generated by the enzyme uridine phosphorylase-1 (UPP1), and we find that neutrophils are a significant source of UPP1 in metastatic cancer. Mammary tumours increase expression of adhesion molecules on the neutrophil surface, in a UPP1-dependent manner, leading to decreased neutrophil motility in the pre-metastatic lung. UPP1-expressing neutrophils suppress T-cell proliferation, and the UPP1 product uracil increases fibronectin deposition in the extracellular microenvironment. Knockout or inhibition of UPP1 in mice with mammary tumours increases T-cell numbers and reduces fibronectin content in the lung, and decreases the proportion of mice that develop lung metastasis. These data indicate that UPP1 influences neutrophil behaviour and extracellular matrix deposition in the lung, and suggest that circulating uracil could be a marker of metastasis, and that pharmacological inhibition of UPP1 could be a strategy to reduce recurrence.
Keywords: Fibronectin; Metastasis; Neutrophils; T Cells; Uridine Phosphorylase.
© 2025. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. The authors declare no competing interests.
Figures



References
-
- Alshetaiwi H, Pervolarakis N, McIntyre LL, Ma D, Nguyen Q, Rath JA, Nee K, Hernandez G, Evans K, Torosian L et al (2020) Defining the emergence of myeloid-derived suppressor cells in breast cancer using single-cell transcriptomics. Sci Immunol 5:eaay6017, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE139125 [DATASET] - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

