Gene therapy ameliorates neuromuscular pathology in CLN3 disease
- PMID: 40702562
- PMCID: PMC12285172
- DOI: 10.1186/s40478-025-02059-z
Gene therapy ameliorates neuromuscular pathology in CLN3 disease
Abstract
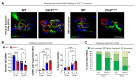
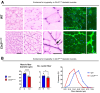
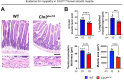
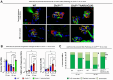
CLN3 disease is a neuronopathic lysosomal storage disorder that severely impacts the central nervous system (CNS) while also inducing notable peripheral neuromuscular symptoms. Although considerable attention has been directed towards the neurodegenerative consequences within the CNS, the involvement of peripheral tissues, including skeletal muscles and their innervation, has been largely neglected. We hypothesized that, CLN3 deficiency could directly influence peripheral nerves and investigated the neuromuscular system in Cln3Δex7/8 mice. Our study found no overt loss of sciatic nerve axons or demyelination in 18-month-old Cln3Δex7/8 mice at disease endstage, but a marked reduction of terminal Schwann cells (tSCs) at lower limb neuromuscular junctions (NMJs), culminating in progressive denervation of these NMJs which appeared abnormal. This led us to investigate skeletal muscle where we found significant myofiber atrophy and decreased and misplaced myofibril nuclei. Similar myopathic alterations were present in a muscle biopsy from an 8-year-old human CLN3 patient shortly after diagnosis. To assess a potential therapeutic intervention, we administered intravenous gene therapy using AAV9.hCLN3 to neonatal Cln3Δex7/8 mice, which at disease endstage, entirely prevented tSC loss and NMJ abnormalities, while also averting skeletal muscle atrophy. These findings underscore the underappreciated, yet substantial effects of CLN3 disease beyond the CNS, highlighting peripheral neuromuscular pathologies as novel features of this disorder. Our findings also indicate that these manifestations could be amenable to treatment via gene therapy, opening new therapeutic strategies in the management of CLN3 disease.
Keywords: AAV9 gene therapy; CLN3 disease; Muscle atrophy; Neuromuscular junction; Peripheral nervous system; Terminal Schwann cells.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: JDC has received research support from BioMarin Pharmaceutical Inc., Abeona Therapeutics Inc., REGENXBIO Inc. and Neurogene, and is a consultant for JCR Pharmaceuticals. ASW received a research grant (unrelated to this work) from Checkpoint Surgical. The remaining authors declare no conflicts of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

