Palladium-Catalyzed Non-Directed C─H Functionalization of Arenes with Trifluoromethylated Olefins
- PMID: 40702805
- PMCID: PMC12376254
- DOI: 10.1002/chem.202501973
Palladium-Catalyzed Non-Directed C─H Functionalization of Arenes with Trifluoromethylated Olefins
Abstract
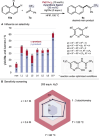
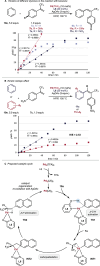
Herein, we report the palladium-catalyzed non-directed C─H functionalization reaction of simple arenes with α-trifluoromethyl styrene derivatives. The application of 2-pyridone ligands increased the yield and selectivity of the alkenylation reaction and enabled a broad scope of different simple arenes and α-trifluoromethyl styrenes (25 examples, up to 94% yield). Overall, a robust reaction is developed as described by a sensitivity screening. Detailed experimental studies on the reaction were performed to further understand the mechanism. Palladium-catalyzed non-directed C─H alkenylation of simple arenes with α-trifluoromethyl styrene derivatives is accomplished using 2-pyridone ligands, which significantly enhances both yield and selectivity. This approach enables efficient functionalization across a wide range of arenes and styrene substrates. Mechanistic studies further clarify the reaction pathway.
Keywords: C─H Functionalization; Pd‐catalysis; fluorine chemistry; trifluoro methylated olefines.
© 2025 The Author(s). Chemistry – A European Journal published by Wiley‐VCH GmbH.
Conflict of interest statement
The author declares no conflict of interest.
Figures


References
-
- a) Dalton T., Faber T., Glorius F., ACS Cent. Sci. 2021, 7, 245; - PMC - PubMed
- b) Wencel‐Delord J., Glorius F., Nat. Chem. 2013, 5, 369; - PubMed
- c) Pei C., Empel C., Koenigs R. M., Angew. Chem., Int. Ed. 2022, 134, 202201743; - PMC - PubMed
- d) Carral‐Menoyo A., Sotomayor N., Lete E., Trends Chem 2022, 4, 495;
- e) Wedi P., van Gemmeren M., Angew. Chem., Int. Ed. 2018, 57, 13016. - PubMed
-
- a) Sambiago S., Schönbauer D., Blieck R., Dao‐Huy T., Porotschnig G., Schaaf G., Wiesinger T., Zia M. F., Wencel‐Delord J., Besset T., Maes B. U. W., Schnürch M. A., Chem. Soc. Rev. 2018, 47, 6603; - PMC - PubMed
- b) Gandeepan P., Ackermann L., Chem 2018, 4, 199;
- c) Lyons T. W., Sanford M. S., Chem. Rev. 2010, 110, 1147; - PMC - PubMed
- d) Neufeld S. R., Sanford M. S., Acc. Chem. Res. 2012, 45, 936; - PMC - PubMed
- e) Rej S., Charani N., Chem. Rev. 2020, 120, 1788. - PubMed
LinkOut - more resources
Full Text Sources

