Unexpected Reactivity of Nitrones: Catalytic Insertion of CS2
- PMID: 40702930
- PMCID: PMC12322958
- DOI: 10.1021/acs.orglett.5c02611
Unexpected Reactivity of Nitrones: Catalytic Insertion of CS2
Abstract
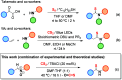
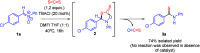
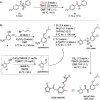
A new reactivity mode for nitrones is reported, consisting of the chloride-catalyzed insertion of CS2 to afford thioamides. This reaction proceeds under metal-free and mild conditions, tolerates a broad family of substrates, and is robust and easily scalable, being successfully applied to the synthesis of the HIV-1 reverse transcriptase inhibitor UC-781. Mechanistic studies, supported by DFT calculations, have revealed the role of chloride anions in the activation of CS2 and its subsequent insertion into nitrones.
Figures


References
-
- Schwalen C. J., Hudson G. A., Kille B., Mitchell D. A.. Bioinformatic Expansion and Discovery of Thiopeptide Antibiotics. J. Am. Chem. Soc. 2018;140:9494–9501. doi: 10.1021/jacs.8b03896. - DOI - PMC - PubMed
- Santos-Aberturas J., Chandra G., Frattaruolo L., Lacret R., Pham T. H., Vior N. M., Eyles T. H., Truman A. W.. Uncovering the Unexplored Diversity of Thioamidated Ribosomal Peptides in Actinobacteria Using the RiPPER Genome Mining Tool. Nucleic Acid Res. 2019;47:4624–4637. doi: 10.1093/nar/gkz192. - DOI - PMC - PubMed
- Liao Y., Zhang S., Jiang X.. Construction of Thioamide Peptides from Chiral Amino Acids. Angew. Chem., Int. Ed. 2023;62:e202303625. doi: 10.1002/anie.202303625. - DOI - PubMed
-
For a general book chapter, see:
- Barrett, T. M. ; Fiore, K. E. ; Liu, C. ; James Petersson, E. In Thioamide-Containing Peptides and Proteins, in Chemistry of Thioamides; Murai, T. , Ed.; Springer: Singapore, 2019.
-
- Kenney G. E., Dassama L. M., Pandelia M.-E., Gizzi A. S., Martinie R. J., Gao P., DeHart C. J., Schachner L. F., Skinner O. S., Ro S. Y.. et al. The Biosynthesis of Methanobactin. Science. 2018;359:1411–1416. doi: 10.1126/science.aap9437. - DOI - PMC - PubMed
- Litomska A., Ishida K., Dunbar K. L., Boettger M., Coyne S., Hertweck C.. Enzymatic Thioamide Formation in a Bacterial Antimetabolite Pathway. Angew. Chem., Int. Ed. 2018;57:11574–11578. doi: 10.1002/anie.201804158. - DOI - PubMed
- Mahanta N., Szantai-Kis D. M., Petersson E. J., Mitchell D. A.. Biosynthesis and Chemical Application of Thioamides. ACS Chem. Biol. 2019;14:142–163. doi: 10.1021/acschembio.8b01022. - DOI - PMC - PubMed
-
-
For a general review, see:
- Jagodzinski T. S.. Thioamides as Useful Synthons in the Synthesis of Heterocycles. Chem. Rev. 2003;103:197. doi: 10.1021/cr0200015. - DOI - PubMed
-
For selected previous examples, see:
- Inamoto K., Hasegawa C., Hiroya K., Doi T.. Palladium-Catalyzed Synthesis of 2-Substituted Benzothiazoles via a C-H Functionalization/Intramolecular C-S Bond Formation Process. Org. Lett. 2008;10:5147–5150. doi: 10.1021/ol802033p. - DOI - PubMed
- Wang H., Wang L., Shang J., Li X., Wang H., Gui J., Lei A.. Fe-Catalysed Oxidative C-H Functionalization/C-S Bond Formation. Chem. Commun. 2012;48:76–78. doi: 10.1039/C1CC16184A. - DOI - PubMed
- Yajima K., Yamaguchi K., Mizuno N.. Facile Access to 3,5-Symmetrically Disubstituted 1,2,4-Thiadiazoles through Phosphovanadomolybdic Acid Catalyzed Aerobic Oxidative Dimerization of Primary Thioamides. Chem. Commun. 2014;50:6748–6750. doi: 10.1039/C4CC02313G. - DOI - PubMed
-
-
- Chen X., Mietlicki-Baase E. G., Barrett T. M., McGrath L. E., Koch-Laskowski K., Ferrie J. J., Hayes M. R., Petersson E. J.. Thioamide Substitution Selectively Modulates Proteolysis and Receptor Activity of Therapeutic Peptide Hormones. J. Am. Chem. Soc. 2017;139:16688–16695. doi: 10.1021/jacs.7b08417. - DOI - PMC - PubMed
- Ghosh P., Raj N., Verma H., Patel M., Chakraborti S., Khatri B., Doreswamy C. M., Anandakumar S. R., Seekallu S., Dinesh M. B., Jadhav G., Yadav P. N., Chatterjee J.. An Amide to Thioamide Substitution Improves the Permeability and Bioavailability of Macrocyclic Peptides. Nat. Commun. 2023;14:6050. doi: 10.1038/s41467-023-41748-y. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

