Turning the Tables: Ligand-Centered Hydride Shuttling in Organometallic BIP-Al Systems
- PMID: 40703003
- PMCID: PMC12326362
- DOI: 10.1021/acs.inorgchem.5c02587
Turning the Tables: Ligand-Centered Hydride Shuttling in Organometallic BIP-Al Systems
Abstract
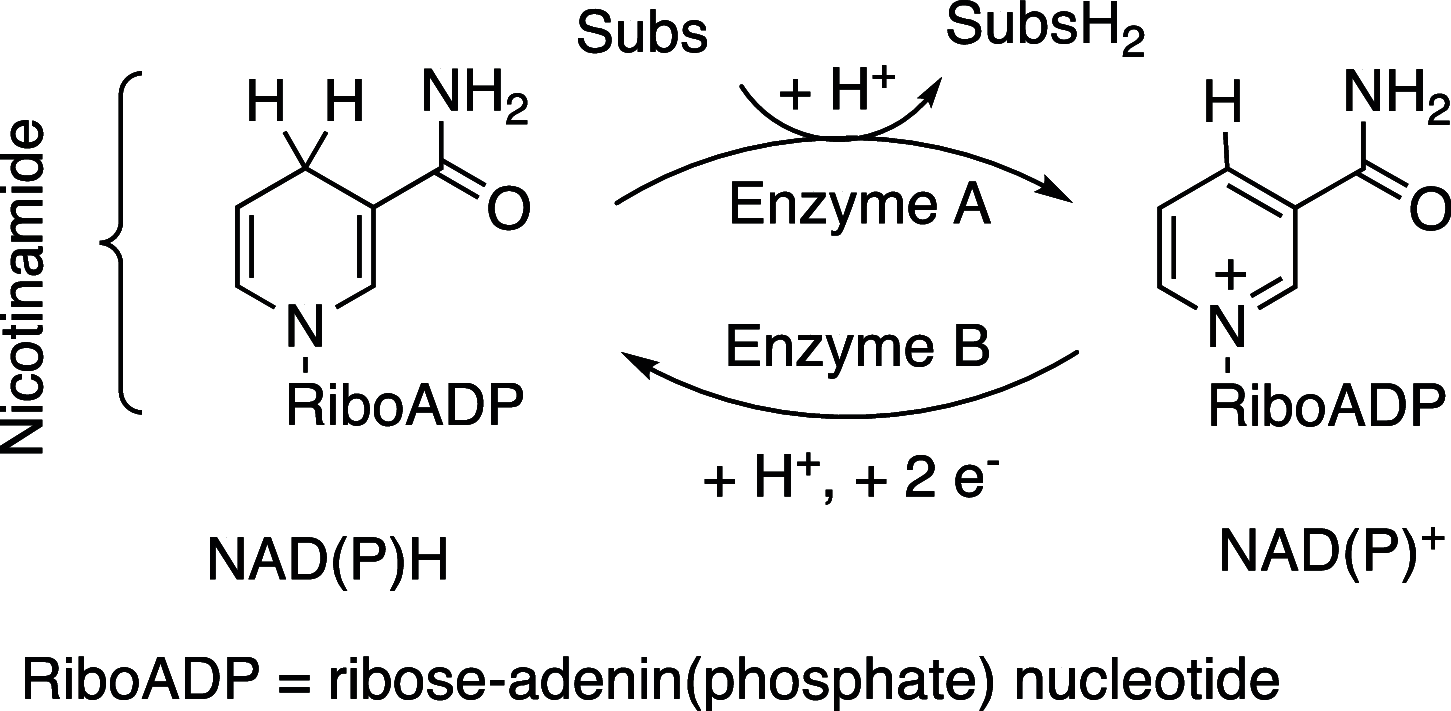
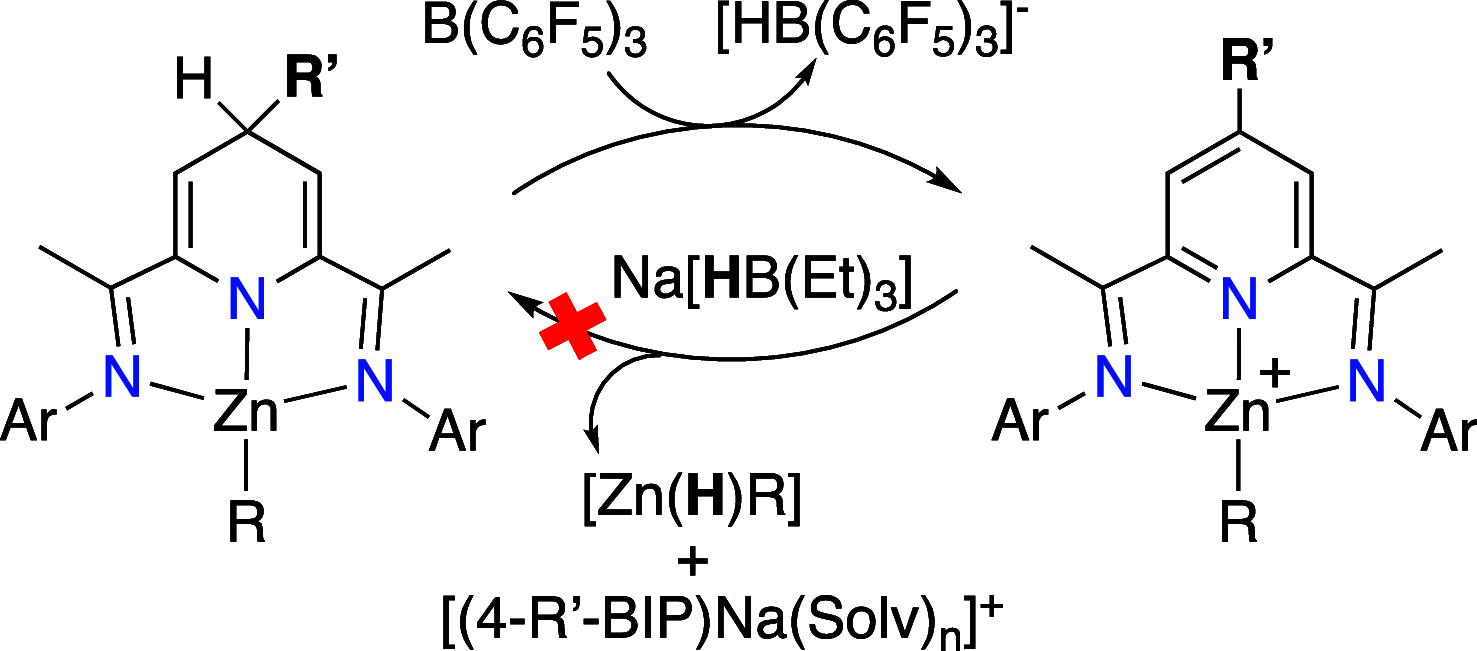
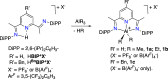
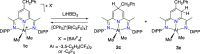
The reversible storage and release of hydride equivalents remains a central challenge in the design of biomimetic redox systems. Cationic 2,6-bis(imino)pyridine organoaluminum complexes [(4-R-BIP)AlR2]+ (where R = H; R' = Me, 1a; R' = Et, 1b; R = Bn; R' = Me, 1c) and their neutral 2,6-bis(imino)-4-R-dihydropyridinate counterparts [(4-R-HBIP)AlR2] 2a-c are presented as chemically reversible hydride exchangers. Interconversion between these systems is achieved through strong reducing agents such as M+[HBEt3]- (where M = Li; Na) or LiAlH4, while powerful electrophiles like B(C6F5)3 or cationic trityl salts Ph3C+ enable the reverse transformation, with the latter providing complete selectivity. Overall, this reversible hydride exchange mirrors natural NAD(P)H/NADP+ cofactor system. These findings establish a new platform for ligand-centered hydride shuttling, where the metal fragment acts as a passive modulator─inverting the traditional roles assigned to metal and ligand.
Figures





References
-
- Sellés Vidal L., Kelly C. L., Mordaka P. M., Heap J. T.. Review of NAD(P)H-dependent oxidoreductases: Properties, engineering and application. Biochim. Biophys. Acta, Proteins Proteomics. 2018;1866:327–347. doi: 10.1016/j.bbapap.2017.11.005. - DOI - PubMed
- Chen M.-W., Wu B., Liu Z., Zhou Y.-G.. Biomimetic Asymmetric Reduction Based on the Regenerable Coenzyme NAD(P)H Models. Acc. Chem. Res. 2023;56:2096–2109. doi: 10.1021/acs.accounts.3c00129. - DOI - PubMed
- Roth S., Niese R., Müller M., Hall M.. Redox Out of the Box: Catalytic Versatility Across NAD(P)H-Dependent Oxidoreductases. Angew. Chem., Int. Ed. 2024;63:e202314740. doi: 10.1002/anie.202314740. - DOI - PubMed
-
- Nelson, D. L. ; Lehninger, A. L. ; Cox, M. M. . Lehninger principles of biochemistry; Macmillan, 2021.
- Berg, J. M. ; Gatto, G. J., Jr ; Hines, J. ; Tymoczko, J. L. ; Stryer, L. . Biochemistry; Macmillan Higher Education, 2023.
- Lippard, S. J. ; Berg, J. M. . Principles of Bioinorganic Chemistry; University Science Books, 1994.
- Ochiai, E. Bioinorganic Chemistry: A Survey; Academic Press, 2008.
-
- Special issues and article collections: Earth-Abundant Metals In Catalysis. In Eur. J. Org. Chem. Eurjoc-Special Collection; Wiley, 2017.
- Berben, L. ; de Bruin, B. . RSC Themed Collection “Earth Abundant Metals in Catalysis”. Edited by.
- Wheelhouse K. M. P., Webster R. L., Beutner G. L.. Organometallics: special issue “Advances and Applications in Catalysis with Earth-Abundant Metals. Editorial article. Organometallics. 2023;42:1677–1679. doi: 10.1021/acs.organomet.3c00292. - DOI
-
- Bellabarba R., Johnston P., Moss S., Sievers C., Subramaniam B., Tway C., Wang Z., Zhu H.. Net Zero Transition: Possible Implications for Catalysis. ACS Catal. 2023;13:7917–7928. doi: 10.1021/acscatal.3c01255. Reviews and books: - DOI
- Su B., Cao Z.-C., Shi Z.-J.. Exploration of Earth-Abundant Transition Metals (Fe, Co, and Ni) as Catalysts in Unreactive Chemical Bond Activations. Acc. Chem. Res. 2015;48:886–896. doi: 10.1021/ar500345f. - DOI - PubMed
- Ni C., Ma X., Yang Z., Roesky H. W.. Recent Advances in Aluminum Compounds for Catalysis. Eur. J. Inorg. Chem. 2022;2022:e202100929. doi: 10.1002/ejic.202100929. - DOI
- Chong E., Wu H., Lee J., Forson K., Haddad N.. Recent Advances in Non-Precious Metal Catalysis. Org. Proc. Res. Develop. 2023;27:1931–1953. doi: 10.1021/acs.oprd.3c00310. - DOI
- Bullock, M. Catalysis without Precious Metals; Wiley, 2010.
LinkOut - more resources
Full Text Sources
Miscellaneous

