Bread crust extract is a novel activator of aryl hydrocarbon receptor and modulator of NRF2 and NFκB in HepG2 and HCT 116 cells
- PMID: 40703149
- PMCID: PMC12284547
- DOI: 10.1016/j.crfs.2025.101144
Bread crust extract is a novel activator of aryl hydrocarbon receptor and modulator of NRF2 and NFκB in HepG2 and HCT 116 cells
Abstract
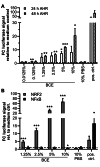
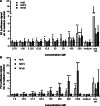
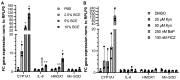
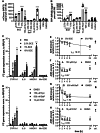
The Maillard reaction describes the non-enzymatic formation of advanced glycation end products (AGEs), e.g., during thermal food processing. Studies on the mode of action and health implications of food-derived AGEs are often contradictory and lack information on active components. We use bread crust extract (BCE) as a model for an AGE-rich diet. Despite the identified AGEs and known activated signaling pathways, it is still unclear which receptors can exert the various effects described for BCE. This study investigates whether BCE can induce the aryl hydrocarbon receptor (AHR), the downstream NRF2 and NFκB signaling pathways and if this activation can be attributed to individual, free AGEs or AHR-agonists present in BCE. HepG2 reporter cell results showed activation of AHR and NRF2 but not NFκB by BCE. However, the tested free AGEs did not show an activation. Known AHR-(pro-)agonists kynurenine (Kyn) and benzo[a]pyrene (BaP), both present in BCE, activated the reporter to a similar extent as BCE with distinct differences in target gene induction of CYP1A1, interleukin-8, heme oxygenase 1 and Manganese-superoxide dismutase. Furthermore, CYP1A1 ethoxyresorufin-O-deethylase enzymatic activity was also induced and could be modulated by AHR and NRF2-inhibition. In contrast, in HCT 116 pTRAF reporter cells, BCE activated AHR, NFκB and NRF2 and induced CYP1A1. We conclude that BCE contains potent AHR activators that influence cellular signaling activities. AHR most likely concerts cell line dependent NRF2 and NFκB-activation. The BCE effects are probably attributable to an interplay of AHR-agonists and AGEs.
Keywords: Advanced glycation end products; Aryl hydrocarbon receptor; Bread crust extract; Maillard reaction; NFκB; NRF2.
© 2025 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Andreas Simm reports financial support was provided by 10.13039/501100001659German Research Foundation. Merve Kuru-Schors reports financial support was provided by 10.13039/501100001659German Research Foundation. Kristin Waechter reports financial support was provided by European Regional Development Fund. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Melatonin and Its Metabolites Can Serve as Agonists on the Aryl Hydrocarbon Receptor and Peroxisome Proliferator-Activated Receptor Gamma.Int J Mol Sci. 2023 Oct 23;24(20):15496. doi: 10.3390/ijms242015496. Int J Mol Sci. 2023. PMID: 37895177 Free PMC article.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Incentives for preventing smoking in children and adolescents.Cochrane Database Syst Rev. 2017 Jun 6;6(6):CD008645. doi: 10.1002/14651858.CD008645.pub3. Cochrane Database Syst Rev. 2017. PMID: 28585288 Free PMC article.
References
-
- Bartling B., Rehbein G., Somoza V., Silber R.E., Simm A. Maillard reaction product‐rich food impair cell proliferation and induce cell death in vitro. Signal Transduct. 2005;5(6):303–313. doi: 10.1002/sita.200500066. - DOI
-
- Behrens A., Schirmer K., Bols N.C., Segner H. Microassay for rapid measurement of 7-ethoxyresorufin-O-deethylase activity in intact fish hepatocytes. Mar. Environ. Res. 1998;46(1):369–373. doi: 10.1016/S0141-1136(97)00039-1. - DOI
LinkOut - more resources
Full Text Sources

