Mitochondria as Regulators of Nonapoptotic Cell Death in Cancer
- PMID: 40703196
- PMCID: PMC12284444
- DOI: 10.1002/mco2.70244
Mitochondria as Regulators of Nonapoptotic Cell Death in Cancer
Abstract
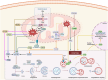
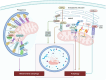
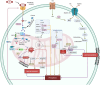
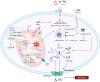
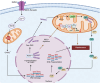
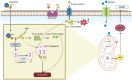
Mitochondria are involved in cell survival and metabolic processes including adenosine triphosphate production, heme biosynthesis, reactive oxygen species, and iron and calcium homeostasis. Although mitochondria are well known to contribute to apoptosis, a growing body of evidence indicates that mitochondria modulate nonapoptotic cell death (NACD) mechanisms, including autophagy, necroptosis, ferroptosis, paraptosis, pyroptosis, parthanatosis, and cuproptosis. These NACD pathways differ in molecular triggers, morphological characteristics, and immunological consequences, but they all involve mitochondria. For example, mitochondrial ROS and lipid peroxidation play a role in ferroptosis, whereas mitochondrial depolarization and the release of apoptosis inducing factor are paramount to parthanatosis. Mitochondrial swelling is a hallmark of paraptosis, whereas mitochondrial disruption is associated with pyroptosis. Autophagy, though primarily a survival mechanism, is also regulated by mitochondrial dynamics in cancer cells. In cuproptosis, mitochondrial protein aggregates when iron-sulfur cluster proteins are disrupted, resulting in copper-dependent cell death. There are many factors that influence NACD, including mitochondrial membrane potential, bioenergetics, calcium flux, metabolites, and interactions with the endoplasmic reticulum. The review comprehensively summarizes our understanding of mitochondrial and NACD interactions, particularly in cells resistant to classical apoptosis agents. Therapeutic vulnerabilities associated with mitochondria-mediated NACD could lead to next-generation therapies.
Keywords: autophagy; cancer; ferroptosis; mitochondria; necroptosis; nonapoptotic cell death.
© 2025 The Author(s). MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Siekevitz P., “Powerhouse of the cell,” Scientific American 197, no. 1 (1957): 131–144.
-
- Vandecasteele G., Szabadkai G., and Rizzuto R., “Mitochondrial Calcium Homeostasis: Mechanisms and Molecules,” Iubmb Life 52, no. 3‐5 (2001): 213–219. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
