Exploring the influence of gut microbiota metabolites on vitiligo through the gut-skin axis
- PMID: 40703229
- PMCID: PMC12283607
- DOI: 10.3389/fmicb.2025.1566267
Exploring the influence of gut microbiota metabolites on vitiligo through the gut-skin axis
Abstract
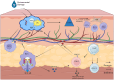
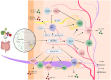
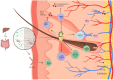
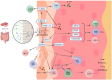
Vitiligo is an autoimmune skin disease with a complex pathogenesis closely linked to immune imbalance and oxidative stress. Currently, comprehensive curative treatments and effective relapse prevention strategies are lacking. Recently, the "gut-skin axis" hypothesis has offered new insights into the pathological mechanisms of vitiligo. Studies indicate that gut microbiota and their metabolic products significantly affect disease progression by regulating immune homeostasis and inflammatory responses in the host. This review systematically examines the effects of short-chain fatty acids, secondary bile acids, and tryptophan metabolites on the human immune system and the inflammatory milieu, and their direct impact on melanocytes. Furthermore, considering the reduced diversity of gut microbiota in individuals with vitiligo, this article also evaluates methods including probiotic intervention, the Mediterranean diet, and fecal microbiota transplantation, which may emerge as potential therapeutic strategies for vitiligo by restoring microbiota balance. Future multidimensional therapeutic strategies that target gut microbiota metabolites show promise for pioneering innovative approaches in vitiligo management.
Keywords: gut microbiota; secondary bile acids; short-chain fatty acids; tryptophan; vitiligo.
Copyright © 2025 Yuan, Liu, Zeng, Yuan, Guo and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Comprehensive single-cell chromatin and transcriptomic profiling of peripheral immune cells in nonsegmental vitiligo.Br J Dermatol. 2025 Jun 20;193(1):115-124. doi: 10.1093/bjd/ljaf041. Br J Dermatol. 2025. PMID: 39888372
-
Role of the intestinal flora-immunity axis in the pathogenesis of rheumatoid arthritis-mechanisms regulating short-chain fatty acids and Th17/Treg homeostasis.Mol Biol Rep. 2025 Jun 21;52(1):617. doi: 10.1007/s11033-025-10714-w. Mol Biol Rep. 2025. PMID: 40544212 Review.
-
Effects of a veterinary gastrointestinal diet on fecal characteristics, metabolites, and microbiota concentrations of adult cats treated with metronidazole.J Anim Sci. 2024 Jan 3;102:skae274. doi: 10.1093/jas/skae274. J Anim Sci. 2024. PMID: 39279199
-
Gut Microbiota-Targeted Therapeutics for Metabolic Disorders: Mechanistic Insights into the Synergy of Probiotic-Fermented Herbal Bioactives.Int J Mol Sci. 2025 Jun 7;26(12):5486. doi: 10.3390/ijms26125486. Int J Mol Sci. 2025. PMID: 40564947 Free PMC article. Review.
-
Gut-Liver Axis: The Role of Intestinal Microbiota and Their Metabolites in the Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease.Gut Liver. 2025 Jul 15;19(4):479-507. doi: 10.5009/gnl240539. Epub 2025 May 8. Gut Liver. 2025. PMID: 40336226 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

