Hemodynamic and neuronal contributions to low-frequency vascular oscillations in a preclinical model of Alzheimer's disease
- PMID: 40703504
- PMCID: PMC12285523
- DOI: 10.1117/1.NPh.12.S1.S14615
Hemodynamic and neuronal contributions to low-frequency vascular oscillations in a preclinical model of Alzheimer's disease
Abstract
Significance: Vasomotion, a temporal oscillation in vascular diameter centered around 0.1 Hz, may be altered in Alzheimer's disease (AD), with both increases and decreases reported.
Aim: We aimed to better characterize vasomotion in vivo, assess its feasibility as an early biomarker for vascular dysfunction in AD, and determine the relationship of vasomotion to underlying neuronal activity.
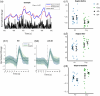
Approach: Low-frequency (0.06 to 0.2 Hz) oscillations (LFOs) in the cerebral arteries of anesthetized 9- to 12-month-old J20-AD ( ) and wild-type ( ) mice were extrapolated from hemodynamic data obtained using 2D optical imaging spectroscopy (2D-OIS). Changes in LFO power were determined after an inspired gas challenge and compared between groups. Simultaneously gathered multi-unit neuronal activity data were used to determine whether LFOs were independent of neural activity.
Results: LFOs increased as inspired oxygen was reduced, but the change in LFO power did not differ between groups. LFOs were found to be driven by neuronal activity, suggesting that they represent spontaneous low-frequency neurovascular coupling rather than vascular-only derived activity.
Conclusions: Arterial LFOs obtained by 2D-OIS were not a suitable metric to distinguish anesthetized J20-AD males from healthy male controls. Furthermore, hemodynamic oscillations occurring within the same frequency range as vasomotion may reflect underlying neuronal activity.
Keywords: Alzheimer’s disease; neurovascular coupling; vasomotion.
© 2025 The Authors.
Figures







References
Associated data
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

