The saline infusion test with mass spectrometric measurements of aldosterone to confirm primary aldosteronism
- PMID: 40704404
- PMCID: PMC12404626
- DOI: 10.1097/HJH.0000000000004098
The saline infusion test with mass spectrometric measurements of aldosterone to confirm primary aldosteronism
Abstract
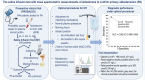
Objective: Confirmation of primary aldosteronism with the saline infusion test requires accurate measurements of plasma aldosterone, which is best achieved by mass spectrometry. Diagnostic performance, appropriate cut-offs and intra-patient variability of the test remain inadequately defined. The objective of this prospective multicenter cohort study was to address these limitations.
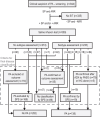
Methods: Primary aldosteronism was confirmed and excluded using alternative criteria to confirmatory tests in 138 and 282 respective patients with suspected disease. Those criteria were not satisfied in 89 patients. Diagnostic performance of the saline infusion test and optimal cut-offs were determined from receiver operating characteristic curves. Intra-patient variability was determined in 57 patients.
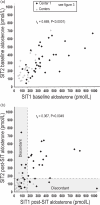
Results: Analysis of receiver operating characteristic curves indicated an area under the curve of 0.964 and a cut-off of 169 pmol/l for posttest aldosterone concentrations that provided 97% sensitivity and 89% specificity. A cut-off of 255 pmol/l enabled improved specificity of 95% at a sensitivity of 75%. Among the 57 patients in whom the saline infusion test was repeated, 15 (26%) had posttest aldosterone concentrations that were discordant using the 169 pmol/l cut-off. Eighty percent of the discordant results were from a single center. With exclusion of that center, which did not minimize ambulation during saline infusion, the area under the curve increased to 0.985 and an optimal cut-off of 169 pmol/l provided 96% specificity and sensitivity.
Conclusion: The seated saline infusion test with mass spectrometric measurements of aldosterone and the cut-offs documented here provides a useful confirmatory test, although this requires adherence to standard-operating procedures.
Keywords: LC-MS/MS; aldosterone; confirmatory test; diagnosis; diagnostic sensitivity; diagnostic specificity; mass spectrometry; primary aldosteronism; receiver operating characteristic curve; saline suppression test.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Scientific support not directly related to this study was provided by DiaSorin Pty Ltd and Australian Medical Research Future Fund (MRFAR000172) to J.Y., the Kurt and Senta Hermann Foundation to S.G., Habilitationsförderung für Frauen der TU Dresden (12/2021–11/2022) to C.P., travel support from the Endocrine Society of Australia to G.E. J. Y Leads the Consumer Engagement Committee of the Primary Aldosteronism Foundation. G.E. consults for Roche Diagnostics. G.E., M.R., F.B., M.S., and C.P. are co-holders of submitted patents (WO-2022171680-A1 and German no. 2383/ 21DE) relevant to this study.
Figures


References
-
- Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2016; 101:1889–1916. - PubMed
-
- Mulatero P, Monticone S, Deinum J, Amar L, Prejbisz A, Zennaro MC, et al. Genetics, prevalence, screening and confirmation of primary aldosteronism: a position statement and consensus of the Working Group on Endocrine Hypertension of The European Society of Hypertension. J Hypertens 2020; 38:1919–1928. - PubMed
-
- Jansen PM, van den Born BJ, Frenkel WJ, de Bruijne EL, Deinum J, Kerstens MN, et al. Test characteristics of the aldosterone-to-renin ratio as a screening test for primary aldosteronism. J Hypertens 2014; 32:115–126. - PubMed
-
- Manolopoulou J, Fischer E, Dietz A, Diederich S, Holmes D, Junnila R, et al. Clinical validation for the aldosterone-to-renin ratio and aldosterone suppression testing using simultaneous fully automated chemiluminescence immunoassays. J Hypertens 2015; 33:2500–2511. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

