Direct Co-Targeting of Bcl-xL and Mcl-1 Exhibits Synergistic Effects in AR-V7-Expressing CRPC Models
- PMID: 40704654
- PMCID: PMC12368576
- DOI: 10.1158/2767-9764.CRC-25-0096
Direct Co-Targeting of Bcl-xL and Mcl-1 Exhibits Synergistic Effects in AR-V7-Expressing CRPC Models
Abstract
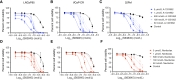
There is an unmet need to develop novel treatment options for patients with metastatic castration-resistant prostate cancer (mCRPC). Patients often develop resistance to next-generation hormonal therapies that target the androgen receptor (AR) axis (e.g., abiraterone and enzalutamide). A splice variant of AR, AR-V7, is associated with resistance to these inhibitors as well as mCRPC progression and poor prognoses. We embarked upon a high-throughput screen to identify synergistic combinations of targeted therapies using two CRPC cell lines, LNCaP95 and VCaP-CR. Combinations targeting BCL2L1 (Bcl-xL) (A-1331852 and navitoclax) and MCL1 (S63845) synergistically decreased cell viability and induced apoptotic activity via cleavage of PARP, caspase 3, and caspase 7 across AR-V7-expressing CRPC cell lines (LNCaP95, VCaP-CR, and 22Rv1) and a patient-derived organoid model (LuCaP 167CR). We also explored the use of a Bcl-xL-specific proteolysis-targeting chimera degrader (PROTAC) to minimize platelet toxicity associated with Bcl-xL inhibitors. We showed similar synergistic efficacy with the Bcl-xL-targeting PROTAC in combination with S63845 in the three-dimensional spheroid models. Our findings support further preclinical development of Bcl-xL and Mcl-1 inhibitors for mCRPC.
Significance: Using an unbiased, combinatorial, high-throughput drug screen, we identified the combination of co-targeting Bcl-xL and Mcl-1 to be highly synergistic across AR-V7-expressing CRPC models. We showed efficacy in higher-order models through validation across in vitro models spanning two-dimensional cell culture, three-dimensional cell culture, and a patient-derived organoid model. These findings identify a promising therapeutic strategy for patients with AR-V7-expressing CRPC.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
A.G. Sowalsky reports grants from Astellas outside the submitted work. No disclosures were reported by the other authors.
Figures





References
-
- Schmidt KT, Huitema ADR, Chau CH, Figg WD. Resistance to second-generation androgen receptor antagonists in prostate cancer. Nat Rev Urol 2021;18:209–26. - PubMed
-
- Li H, Wang Z, Tang K, Zhou H, Liu H, Yan L, et al. Prognostic value of androgen receptor splice variant 7 in the treatment of castration-resistant prostate cancer with next generation androgen receptor signal inhibition: a systematic review and meta-analysis. Eur Urol Focus 2018;4:529–39. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

